Chapter 14: Chemical Kinetics
Kinetics
Kinetics is the study of the change in the [concentrations] of reactants and products over a period of time.
How fast a reaction occurs
[Reactant] or [Product]
Collision Theory Test
In order for a reaction to take place, three things must be true
1. Reacting particles must collide
2. Reactants much collide in the proper orientation
3. Reactants must have sufficient energy, must have minimum amount of energy to react.
The more often they happen = the faster they happen
When all three are true at the same time, they are referred to as an effective collision/successful collision = results in a reaction.
If you increase the concentration of reactants, this will speed up the reactions and lead to more collisions.
Using Collision Theory to Explain Observations Test
Variable/factor that affects reaction | Result | Connection to collision theory |
Increase reactants [R] | More particles in a container will increases reaction rate More frequent and forceful collisions | More particles means more collisions between particles (higher chance of successful collision) |
Increased surface area of solid reactant | Make interior atoms more accessible for collision = increases reaction rate. More surface area | More possible atoms that are accessible, more collisions |
Add a catalyst | a. Catalysts holds a particle in proper orientation (faster reaction) b. Lower activation energy | It will speed up because there are more molecules in the right orientation |
Increase temperature | a. Makes more molecules move faster means a faster reaction b. Gives individual molecules more energy | More possibility of collision and more energy required to react. |
Effect of Proper Orientation on Reaction Rate
Catalyst: a substance that increases the rate of a chemical reaction without itself undergoing any permanent change.
To speed up a reaction, you must:
Increase the number of collisions
OR add a catalyst - holds molecules in correct orientation
“Effective” collision means reactants DO convert into products.
Effect of Energy on Reaction Rate
Increasing temperature increases rate of reaction
Increasing temperature increases the speed at which molecules are moving.
2 consequences
When particles move master, there is a higher probability of collision.
When temperature goes up, the energy of the particles go up as well.
Activation Energy
Reaction energy diagrams show how the energy of a reaction changes as reactants are converted into products.
Activation energy (Ea) is the minimum energy required for a chemical reaction to occur, enabling the reactants to overcome the energy barrier and form products.
This energy barrier is represented in the reaction energy diagram as the peak of the curve, illustrating the energy difference between the reactants and the activated complex.
The lower the activation energy, the faster the reaction because there is a higher probability that molecules will have enough energy to react.
Ea = ETransition State - EReactants
Large Ea = more energy needed
Small Ea = less energy needed
Catalysts
Catalysts are a substance that increases the rate of a chemical reactions without itself undergoing any permanent change.
Holds molecules in proper orientation.
Catalysts work by providing a pathway from reactants to products.
Rate
Rate is always a positive value.
Rate: How much a quantity changes over a period of time.
Reaction Rates
Reaction Rate: The speed at which reactants are converted into products over time
Always positive numbers
Rate = \Delta [A] / \Delta t where [A]) is the concentration of reactant A and t is time.
Product = “Appearance”
Reactant = “Disappearance”
Initial formula for reactant will be positive, but the overall rate will be positive.
Question: Why does the reaction rate go down over time? Test
Over time, reactants go down and products go up.
Rates will go down because when there are less reactants, there are less collisions between reactive molecules.
Rate does not stay constant due to decreasing reactants and increasing products.
Reaction Rates Changes Overtime
As time goes on, the rate of a reaction generally slows down.
As time goes on, the [R] decreases.
With fewer reactants present, there are fewer collisions between reactants (fewer reactions).
At some point, the reaction stops because the reactants ran out or because the system has reached equilibrium.
Expressing Reaction Rates
Relative = compare
Relative rate law/equations
What we use when we want to compare the rate of 1 compound to the rate of another compound in the SAME CHEMICAL EQUATION.
When do we need relative rate law?
When you have TIME and CONCENTRATION with 1 COMPOUND use rate equation.
When you have TIME and CONCENTRATION of 2 COMPOUNDS use rate law and THEN relative rate law.
Rate laws
Sometimes referred to as rate equations.
Relates the reaction with TEMPERATURE (affects energy) and the CONCENTRATION (affects number of collisions) of reactants.
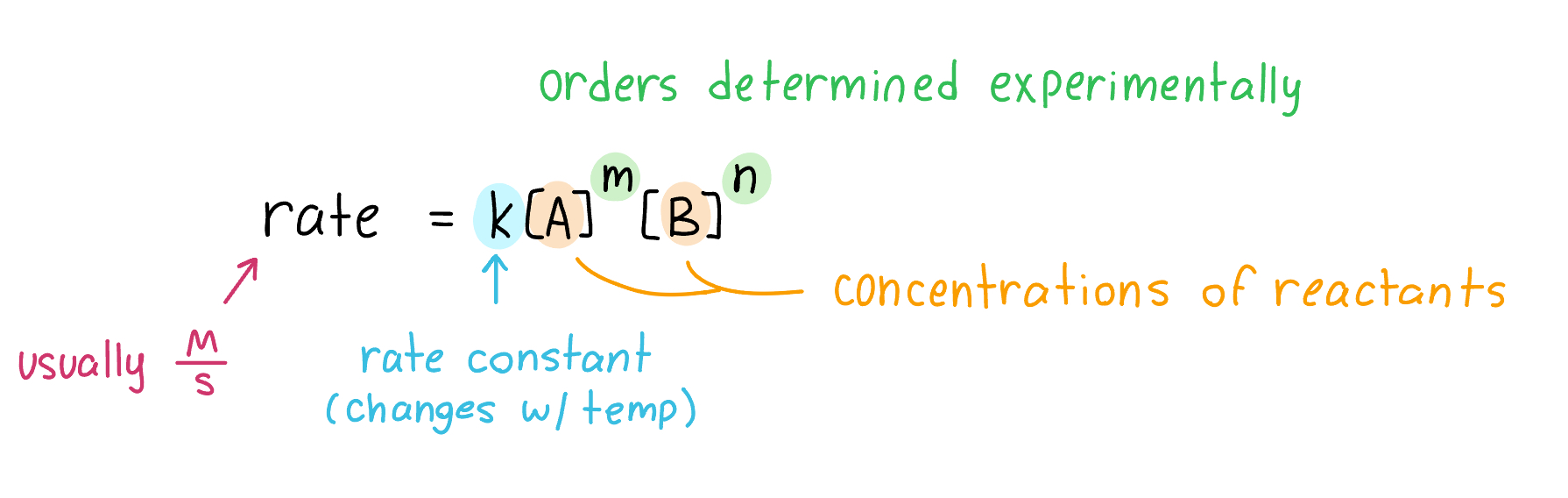
For the general reaction: aA + bB → cC + dD
The rate law is: rate = k[A]m[B]n
Where k is the rate constant (usually M/s) that is specific to the reaction and varies with temperature.
Reaction Order Test
The exponents in rate laws
The sum of the exponents on the reactants is the overall order of reaction.
Order is NOT coefficients
Order tells us how much the rate will increase when we increase the concentration of reactants
If n=0
Rate = k[A]0
Rate is independent to concentration
Rate won’t change even when we change the concentration of that compound
If n=1
Rate = k[A]1
Rate is proportional to the concentration of A
What you do to the concentration of the rate will change it in the same way (if you double/triple the concentration, the rate will double/triple)
If n=2
Rate = k[A]
Rate is proportional to the square
Whatever you do to the concentration the rate will change by the factor of the square (multiply by 2, rate increases by 4, multiply by 5, rate increases by 25)
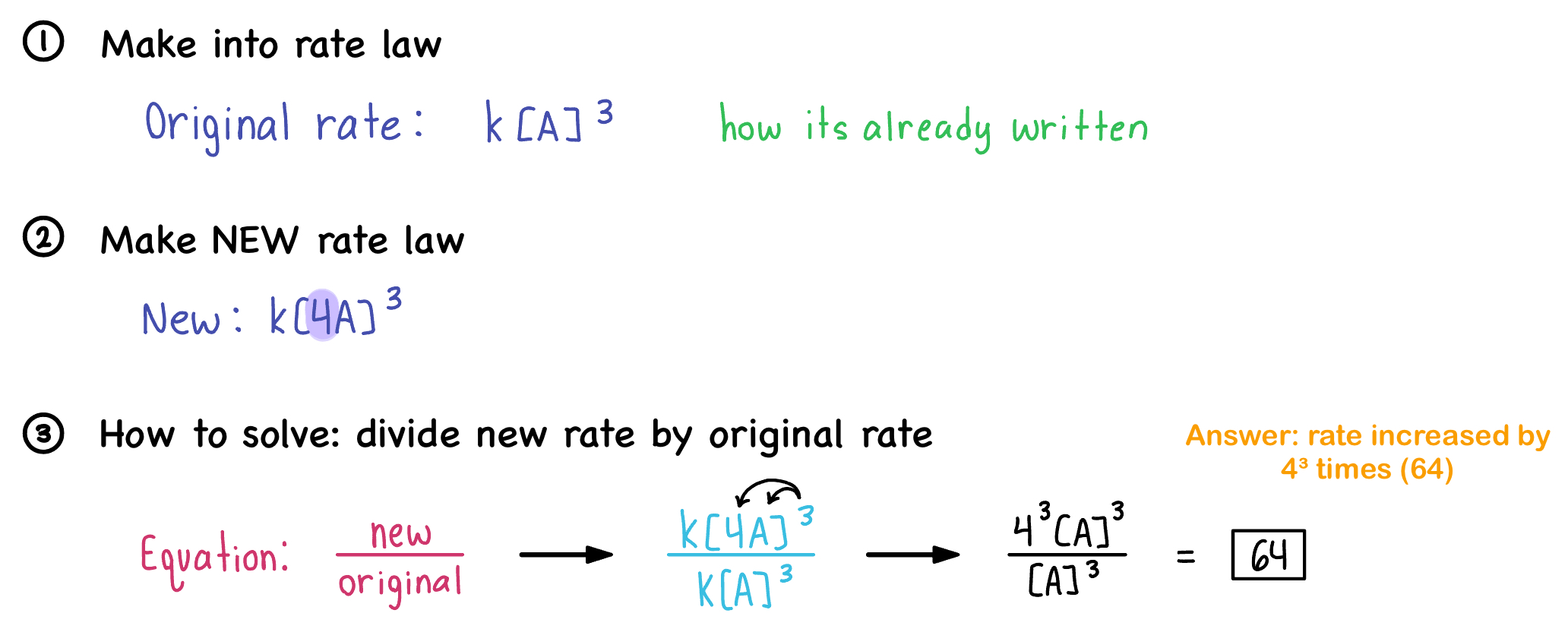
Formula for change in reaction law:
\dfrac{new\ rate}{old\ or \ original\ rate}
Example
In a reaction of third order in A, increasing the concentration of A by a factor of 4 will cause what kind of change in reaction rate?
Determining order from experimental data formula:
\dfrac{rate_{2}}{rate_{1}}=\dfrac{k\left[ A\right]_{2}^{n}}{k\left[ A\right] _{1}^{n}}
Given data is Concentration and Initial Rate M/s
Rate Constant, k (Units)
Zero order = M/s or Ms-1
First order = 1/s or s-1
Second order = M-1/s or M-1s-1
Determining Order for MULTIPLE Reactants
Step 1: Write generic rate law
Step 2: To solve for m
Choose 2 rows where [A] changes, but [B] stays the same.
Step 3 (If you can’t solve for m): Solve for n
Choose 2 rows where [B] changes, but [A] stays the same.
Step 4 (if there was no m): Solve for n
Plug n back into generic rate law
Choose 2 rows where [A] changes (even if [B] changes)
Step 5: Solve for k using rate law
Integrated Rate Law
Portray the relationship between concentration of reactants and time.
When do I use an integrated rate law?
Word problems, no table
Has time and [ ]
Which rate law should I use?
Different integrated rate law for each order of reaction.
The values of these variables are ALWAYS POSITIVE
Half-life
The length of time it takes for half of the reactant to get used up.
It depends on the order of the reaction and k
REMEMBER: when using percentage use as a whole number in the calculations
REMAINS vs DECOMPOSED
Remains: Given % → [A]t
Decomposed: = 100% - given % → [A]t
Assume [A]0 is 100%
When given time
Determining Order of Reaction When Given t and [ ]
When given time (t) and concentration [A], determining order by creating 3 graphs and identifying which is most linear.
Most linear graph will be that order
Zero order: [A]
First order: ln[A]
Second order: 1/[A]
Effect of Temperature on Reaction Rates
For many reactions, increasing temperature by 10°C doubles the rate of reaction.
Rate increases but so does the values of k
Arrhenius equation
Shows the relationship between temperature and the rate constant, k.
Formula on equation sheet
 Knowt
Knowt
