Matter (OCR)
Properties of matter
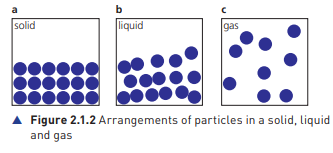
Solids
Structure: Particles in solids are arranged in a highly ordered and fixed pattern, forming a crystalline lattice. This structure is rigid and maintains the shape of the solid.
Intermolecular Forces: Strong intermolecular forces hold the particles closely together, restricting their movement.
Movement: Particles in a solid can only vibrate about their fixed positions.
Properties of Solids:
Definite Shape: Solids maintain their shape regardless of the container they are in.
Definite Volume: Solids have a fixed volume and are not easily compressed.
Incompressibility: Due to the closely packed particles, solids are almost incompressible.
High Density: Solids generally have higher densities compared to liquids and gases because their particles are tightly packed.
Liquids
Structure: Particles in liquids are still close together but not in a fixed arrangement. They can move past each other, allowing liquids to flow.
Intermolecular Forces: Intermolecular forces are weaker than in solids but still significant enough to keep the particles in close proximity.
Movement: Particles can move around and slide past one another, allowing liquids to change shape while maintaining volume.
Properties of Liquids:
Indefinite Shape: Liquids take the shape of their container.
Definite Volume: Liquids have a fixed volume and are not easily compressed.
Fluidity: Liquids can flow and are considered fluids.
Moderate Density: Liquids have densities lower than solids but higher than gases.
Gases
Structure: Particles in gases are far apart compared to solids and liquids. There is no fixed arrangement, and particles move freely.
Intermolecular Forces: Very weak intermolecular forces exist between gas particles, allowing them to move independently.
Movement: Gas particles move rapidly and randomly in all directions.
Properties:
Indefinite Shape and Volume: Gases expand to fill the shape and volume of their container.
Compressibility: Gases are easily compressed because of the large spaces between particles.
Low Density: Gases have much lower densities compared to solids and liquids.
Changes of State
Melting (Solid to Liquid)
When a solid is heated, its particles gain energy and vibrate more vigorously until they overcome the strong intermolecular forces holding them in place. The temperature at which this occurs is the melting point.
Freezing (Liquid to Solid)
When a liquid is cooled, its particles lose energy and move less, allowing the intermolecular forces to arrange them into a fixed, orderly structure. The temperature at which this happens is the freezing point.
Boiling (Liquid to Gas)
When a liquid is heated, its particles gain enough energy to overcome the intermolecular forces holding them together. They break free and form a gas. The temperature at which this occurs is the boiling point.
Condensation (Gas to Liquid)
When a gas is cooled, its particles lose energy and move closer together, forming a liquid as intermolecular forces pull them together.
Sublimation (Solid to Gas)
In some substances, particles gain enough energy to transition directly from solid to gas without passing through the liquid phase.
Deposition (Gas to Solid)
Gas particles lose enough energy to transition directly into a solid without passing through the liquid phase.
Density
⇾ Density is the measure of how compact the mass in an object/substance is
⇾ Mass per unit volume
⇾ SI unit: kg/m³
⇾ ρ = m/v
⤷ ρ: density
⤷ m: mass
⤷ v: volume
Buoyancy: Objects with lower density than a fluid will float in that fluid.
Examples:
Water: Density of water is approximately 1000 kg/m³ (1 g/cm³).
Air: Density of air at sea level is about 1.225 kg/m³.
Iron: Density of iron is about 7874 kg/m³.
Pressure
Pressure (P) is the force exerted per unit area.
P=FAP = \frac{F}{A}P=AF
P: Pressure (Pascals, Pa)
F: Force (Newtons, N)
A: Area (square meters, m²)
The SI unit of pressure is the Pascal (Pa)
Other units include atmospheres (atm), millimeters of mercury (mmHg), and pounds per square inch (psi).
Types of Pressure:
Atmospheric Pressure: The pressure exerted by the weight of the atmosphere. At sea level, it is approximately 101,325 Pa (1 atm).
Gauge Pressure: The pressure relative to atmospheric pressure.
Absolute Pressure: The total pressure including atmospheric pressure.
Pressure in Fluids:
Hydrostatic Pressure: The pressure in a fluid at a given depth due to the weight of the fluid above it.
Formula: P=P0+ρghP = P_0 + \rho ghP=P0+ρgh
P0P_0P0: Atmospheric pressure (at the surface)
ρ\rhoρ: Density of the fluid
ggg: Acceleration due to gravity (9.8 m/s²)
hhh: Depth below the surface of the fluid
Applications:
Hydraulics: Systems that use fluid pressure to transmit force (e.g., hydraulic presses).
Weather Forecasting: Atmospheric pressure is a key factor in weather patterns and forecasts.
Diving: Pressure increases with depth underwater, affecting the divers and their equipment.
Boyle's Law:
Describes the inverse relationship between pressure and volume for a fixed amount of gas at a constant temperature.
Formula: P1V1=P2V2P_1 V_1 = P_2 V_2P1V1=P2V2
P1,P2P_1, P_2P1,P2: Initial and final pressures
V1,V2V_1, V_2V1,V2: Initial and final volumes
Measurement:
Manometer: Measures the pressure of gases or liquids.
Barometer: Measures atmospheric pressure.
Examples:
Syringe: Pushing the plunger increases pressure, which can be used to draw fluid into the syringe.
Tire Pressure: Maintaining the correct pressure in vehicle tires is essential for safety and performance.
Pressure in Solids:
In solids, pressure can be applied and distributed over an area.
Stress: Related to pressure, it is the force per unit area within materials.
Formula: Stress=FA\text{Stress} = \frac{F}{A}Stress=AF
Pascal's Principle:
In a fluid, pressure applied to any point is transmitted undiminished throughout the fluid.
Applications: Hydraulic lifts and brakes use Pascal’s principle to amplify force.
Kinetic Theory of Gases
. Kinetic Molecular Theory
Describes the behavior of gases in terms of particles in motion.
Assumptions:
Gas particles are in constant, random motion.
The volume of the gas particles is negligible compared to the volume of the container.
No intermolecular forces act between the particles.
Collisions between gas particles and with the container walls are perfectly elastic.
Ideal Gas Law
Equation: PV=nRTPV = nRTPV=nRT
PPP: Pressure
VVV: Volume
nnn: Number of moles
RRR: Universal gas constant (8.31 J/mol·K)
TTT: Temperature in Kelvin
. Boltzmann Constant
Relates the average kinetic energy of particles in a gas to the temperature of the gas.
Formula: $$Ek=32kBTE_k = \frac{3}{2} k_B TEk=23kBT
EkE_kEk: Average kinetic energy
kBk_BkB: Boltzmann constant (1.38 × 10⁻²³ J/K)
TTT: Temperature in Kelvin
Thermal Properties of Matter
Temperature and Heat
Temperature: Measure of the average kinetic energy of the particles in a substance.
Heat: Form of energy transfer due to temperature difference.
Specific Heat Capacity
Amount of heat required to raise the temperature of 1 kg of a substance by 1°C.
Formula: Q=mcΔTQ = mc\Delta TQ=mcΔT
QQQ: Heat energy
mmm: Mass
ccc: Specific heat capacity
ΔT\Delta TΔT: Temperature change
Units: J/(kg·°C)
Specific Latent Heat
Heat energy required to change the state of 1 kg of a substance without a change in temperature.
Formula: Q=mLQ = mLQ=mL
QQQ: Heat energy
mmm: Mass
LLL: Specific latent heat
Types:
Latent heat of fusion: Energy required for solid to liquid.
Latent heat of vaporization: Energy required for liquid to gas.
Units: J/kg
Internal Energy
Total energy stored in the particles of a substance.
Sum of kinetic energy (due to particle motion) and potential energy (due to particle interactions).
Change in Internal Energy
Change in internal energy (ΔU\Delta UΔU) due to heat added (QQQ) and work done (WWW):
Formula: ΔU=Q−W\Delta U = Q - WΔU=Q−W
Ideal Gases and Real Gases
Ideal Gases
Hypothetical gases that perfectly follow the ideal gas law.
Real Gases
Deviate from ideal behavior at high pressures and low temperatures.
Intermolecular forces and the volume of gas particles become significant.