Protein Targeting and Secretory Pathways Lecture Notes
Overview
By the end of this lecture, you should be able to:
Differentiate between protein targeting in prokaryotic and eukaryotic cells.
Elucidate the significance of cell compartmentalization in protein sorting and function.
Describe the components and functions of the endomembrane system.
Explain the roles of transport signals and pathways in directing proteins to their correct locations.
Detail the molecular machinery, including signal recognition particles (SRPs) and translocons, involved in protein transport.
Central Dogma of Molecular Biology Refresh
DNA is faithfully copied to DNA (replication).
DNA is transcribed to RNA (transcription).
mRNA is translated into protein (translation).
Key question: How do proteins reach their designated locations within the cell to perform their specific functions?
This lecture focuses on protein targeting and secretory pathways, with a focus on proteins secreted out of the cell.
Prokaryotic vs. Eukaryotic Cells
Major difference: presence of a membrane-bound nucleus in eukaryotic cells, which is absent in prokaryotic cells.
Eukaryotic cells possess various organelles, each enclosed by a membrane, whereas prokaryotic cells lack these membrane-bound organelles.
Cell Compartmentalization
Eukaryotic cells exhibit extensive compartmentalization via numerous organelles.
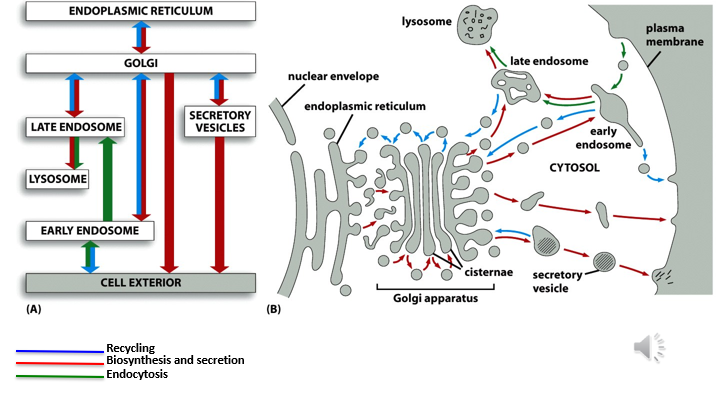
Endoplasmic reticulum (ER): crucial for protein translation, folding, and modification in eukaryotes; site of synthesis for lipids and steroids.
Golgi body/apparatus: involved in protein processing, glycosylation, and packaging into vesicles for transport.
Endosomes, lysosomes, secretory vesicles: each performs specialized functions, such as degradation of cellular waste (lysosomes) and sorting of proteins (endosomes).
Protein trafficking and secretory pathways are more intricate in eukaryotic cells due to their complex organelle structure.
Proteins synthesized in the ER undergo processing in the Golgi apparatus and are then transported to their functional locations through protein trafficking pathways.
Proteins can be recycled between different organelles to ensure proper localization and function.
Organelles possess specialized membranes composed of specific phospholipids and structural/functional proteins that define their identity and function.
Historical Perspective: Golgi Apparatus
Discovered by Camilio Golgi using a unique staining method.
Golgi identified a basket-like structure near the nucleus of Purkinje cells.
The Golgi apparatus plays a critical role in post-translational modification of proteins, including glycosylation and phosphorylation.
Protein Transport
Proteins synthesized in the ER can be transported to the Golgi apparatus for further processing and modification.
Proteins are often incorporated into vesicles for targeted transport to specific cellular locations.
Proteins can be transported into the nucleus through nuclear pores, which facilitate the selective import and export of molecules.
Protein transport across membranes typically involves vesicle-mediated mechanisms to maintain the integrity of cellular compartments.
Molecular Clues
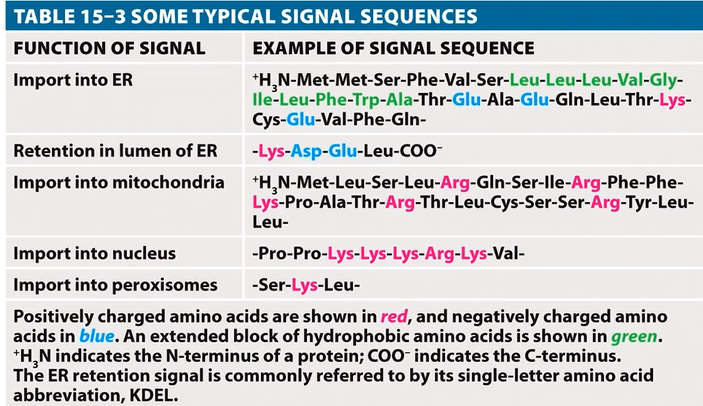
Proteins contain intrinsic molecular clues, such as specific amino acid sequences, that act as targeting signals.
These signals are often located at the N-terminal region of the protein and are highly conserved.
These molecular addresses guide proteins to their correct destinations within the cell.
Examples of protein targeting signals:
Import into the ER: directs proteins to the endoplasmic reticulum for synthesis and modification.
Retention in the ER lumen: ensures proteins remain within the ER for their function.
Import into the mitochondria: targets proteins to the mitochondria for energy production and other metabolic processes.
Import into the nucleus: directs proteins to the nucleus for gene regulation and DNA replication.
Import into peroxisomes: targets proteins to peroxisomes for lipid metabolism and detoxification.
Signal Peptide
Signal peptide: an N-terminal molecular address that guides proteins to the ER membrane.
Located at the N terminus of the protein, usually cleaved off after the protein reaches its destination.
Composed of three distinct regions:
N-terminal region: contains positively charged amino acids (1-5) that facilitate interaction with the negatively charged ER membrane.
Middle hydrophobic region (H region): consists of 7-15 hydrophobic amino acids, including at least three leucines, which promote insertion into the lipid bilayer; tends to form an alpha-helical structure.
C-terminal region: contains 3-7 uncharged amino acids; tends to form a beta-sheet structure that aids in signal peptide recognition and cleavage.
Cleavage site: located between the C region and a negatively charged region, cleaved by a signal peptidase enzyme.
Consensus structure: characterized by the N, H, and C regions with conserved features.
The hydrophobic region is essential for membrane insertion and signal peptide function.
Signal peptidase cleaves the signal peptide, which is initially membrane-anchored through its hydrophobic region.
Protein Trafficking and Secretory Pathways
Approximately 50% of proteins synthesized in the cell are trafficked to specific organelles or secreted outside the cell.
Secretory pathways are crucial for proteins located in the extracellular space, such as those found in blood, cerebrospinal fluid, and the lymphatic system.
There is some variability in the literature regarding the proportion of secreted proteins.
About 34% of genes encode a signal peptide, indicating their involvement in secretory pathways.
Bioinformatics analyses predict that approximately 9% of proteins are secreted (just under 2,000 proteins).
Secretory pathways converge across at least 10 cellular compartments, including the ER, Golgi, and plasma membrane.
The process requires the endomembrane system and specific ligand-receptor interactions to ensure accurate targeting.
Ligand-receptor interactions are analogous to a lock and key, where specific ligands bind to corresponding receptors to initiate transport.
Vesicle Formation
Protein transport is facilitated by the formation of vesicles, which bud off from donor membranes and fuse with acceptor membranes.
Vesicles transport protein cargo to its designated location, ensuring proper delivery.
Specific vesicles are generated based on donor-acceptor vesicle interactions, which are mediated by targeting signals.
Receptor-ligand interaction determines the destination of the vesicle, ensuring it fuses with the correct target membrane.
Proteins on the surface of the vesicles interact with receptors or ligands on the acceptor site, guiding the vesicle to its destination. Vesicles budding off from the Golgi going to the ER (pale blue). Red-coated vesicles traffic proteins toward the Golgi.
Vesicle Transport Proteins
Clathrin: a specific protein involved in vesicle formation, particularly in endocytosis and transport from the Golgi apparatus.
COP1 and COP2: coat protein complexes that mediate vesicle transport between the ER and the Golgi apparatus.
Analogy: Vesicles function like "molecular taxis," delivering proteins to their correct locations within the cell.
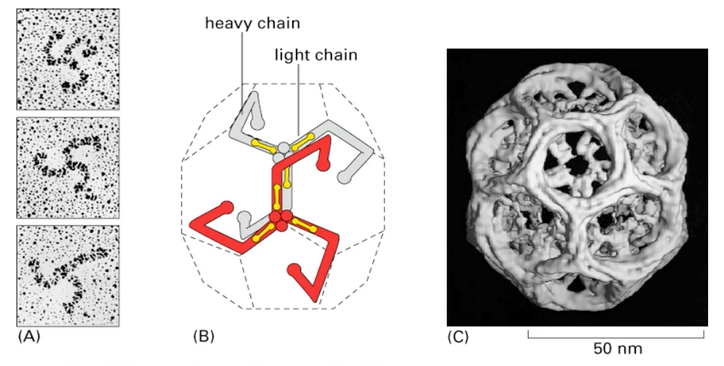
Calthrin- 2 in each molecule
cytoskeleton is vital for vesicle transport-travel along the fibres
vesicles can be transported along microtubules
Motor proteins cytoskeleton- intracellular motorways
kinesin ,dynein- motor protein
Bind to microtubule and vesicle
Dynein- minus end of microtubule
Kinesin- plus end of microtubule
Mutations
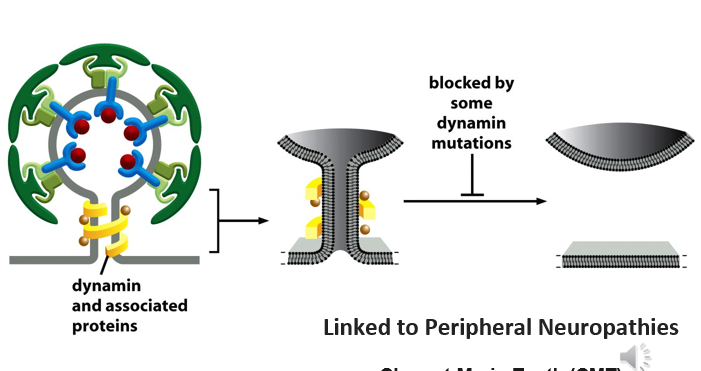
dynamin large gtp aise - triggers release of vesicle from membrane
mutation un dynamin prevents this from happening
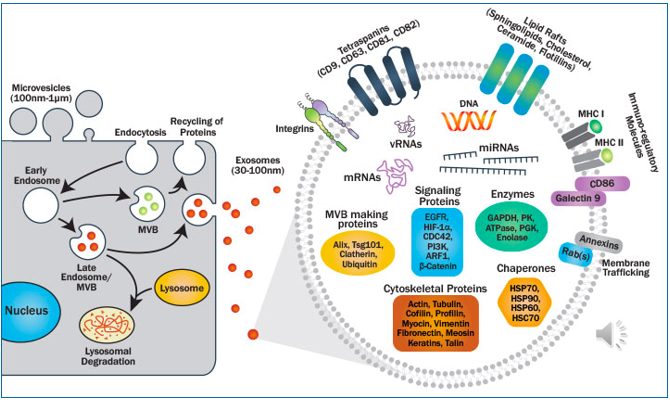
Cell environment communication exosomes