Chemistry: Chapter 2
Atoms, Molecules, and Ions
Atomic Theory
Atoms were known to exist in the 5th century BCE. However, the Atomic Theory was coined by Dalton hundreds of years later in the 1800s.
Lavoisier’s Law of Conservation of Mass (1789): Matter is neither gained nor lost during a chemical reaction.
Proust’s Law of Definite Proportions (1794): All samples of a pure compound contain the same elements in a same fixed proportion by mass.
Ex. All mater molecules have 2 hydrogens atoms and 1 oxygen atom with 11.2% hydrogen and 88.8% oxygen.
Dalton’s Law of Multiple Proportions (1804):
The same elements can be combined in different proportions to form different compounds. The ratios of these compounds will be in a fixed whole number.
For example, 1.116 grams of chlorine and 1 gram of copper will form cupric chloride. But, if only .558 grams of chlorine are added to 1 gram of copper, cuprous chloride (a different compound) will be formed. The elements of cupric chloride and the elements of cuprous chloride will be in a fixed ratio, 2:1.
Dalton’s Atomic Theory
Dalton combined these 5 laws to create the atomic theory:
Matter is composed of exceedingly small particles called atoms. An atom is the smallest unit of an element that can participate in chemical change.
An element consists of only one type of atom, which has a mass that is characteristic to that element and is the same for all of that element’s atoms.
The atoms of one element are unique and have different properties than every other element’s atom.
A compound consists of combined 2 or more atoms that are in a small, whole number ratio and that is the ratio that the atoms will always be in for that particular compound.
Atoms are neither created nor destroyed during a chemical change, just rearranged to create different types of matter.
Practice Question
There are three compounds that contain tin and oxygen. Which of these three are the same compound?
Sample 1: 11.9 grams of tin & 1.6 grams of oxygen
Sample 2: 89 grams of tin and 12 grams of oxygen
Sample 3: 59 grams of tin and 16 grams of oxygen
Remember the Law of Definite Proportions. Two samples that are of the same compound should have the same ratio of one element to the other.
11.9/1.6 = 7.437
89/12 = 7.41667
59/16 = 3.6875
So, the first to samples are the same compound while the third sample is a different compound.
The Discovery of the Electron
JJ Thomson used a high voltage beam to cut a metal sample. He knew the beam was not light because it could move matter so it must contain an unknown particle that is smaller than atoms.
The beam always moved from the negative pole to the positive pole of a batter and could be deflected by a magnet so it must have been negatively charged. The particles are electrons!
But he knew that atoms have a neutral charge so there must be some positive particles that balance out the negative particles. He proposed the “plum pudding” model of an atom.

He also determined the electron’s charge to mass ratio: 1.759 × 1011 C/kg
Defining the Electron
Robert Millikan observed charged oil drops and determined that the charge of an electron is always a multiple of 1.6 × 10-19 C. Thus, using the ratio determined by JJ. Thomson, Millikan found that the mass of an electron is 9.107 × 10-31 kg.
This mass is so small that it does not need to be considered when finding the mass of an atom.
Discovering the Nucleus
In 1911, Rutherford, Geiger, and Marsden shot alpha particles (small, charged particles created by the nuclear decay of radium) at a thin gold foil. While some particles passed straight through the foil, some deflected to the side, and some bounced almost straight back.
The deflected particles indicate a presence of positive charge and the bouncing back of particles indicated a very dense matter. So, an atom must contain a very small, very dense, positive particle: the nucleus.
Atomic Number
The number of protons in the nucleus is called the atomic number.
This is the defining trait of an atom because every atom has a unique number of protons. A neutral atom has an equal number of protons and electrons.
Therefore, the atomic number of a neutral atom also indicates the number of electrons.
Mass Number
In order to find the number of neutrons, the mass number is used. The mass number is the mass of an atom, and it is calculated by adding the number of protons and neutrons in an atom.
Ions
Atoms are usually electrically neutral. When the number of electrons and protons are NOT equal, atoms are electrically charged. Electrically charged atoms are called ions.
The charge of an atom = the number of protons - the number of neutrons
Atoms acquire a net charge by losing or gaining electrons. Ions are either cations or anions.
It is important to remember that ions do not exist separately in our world. Cations and anions must exist together to neutralize each other’s charges.
Formation of Ions
Atoms become positively charged when they lose an electron (because an electron is negative). These positive atoms are called cations.
The atom that receives that electron is called an anion and is negatively charged.
Isotopes
Isotopes are atoms of the same element that have different mass numbers. Isotopes have the same number of protons, but a different number of neutrons. They still have the same number of electrons, so their properties are mostly the same.
Isotope Notation/Symbolism
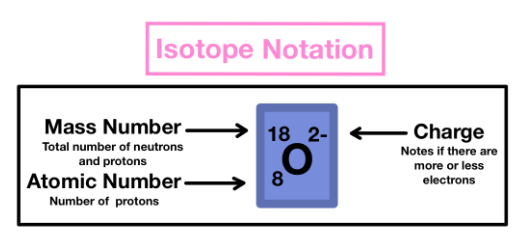
Practice
From this picture, what is the number of:
protons: 8 protons
neutrons: 10 neutrons
electrons: 10 electrons
Radioactive Isotopes
Not all isotopes are radioactive, but some are. Radioactive isotopes are isotopes in which the nucleus spontaneously decays. If a radioactive isotope’s nucleus continues to decay for a long time, it may lose a proton entirely and become another element.
Carbon 14 is a radioactive isotope
Atomic Mass
The atomic mass is the average mass for all isotopes of that element, in proportion to how much of the isotope there is in the world. This is why the atomic mass on the periodic table for each element isn’t exactly the number of neutrons + the number of protons.
Ex. Carbon’s atomic mass on the periodic table is 12.01 amu.
Calculating Atomic Mass
average mass = Σ(fractional abundance x isotopic mass)
this means that the fractional abundance x isotopic mass will be calculated for each isotope and then added together.
The isotopic mass is the mass of each specific isotope. The fractional abundance is the percentage of each isotope that is found in the world.
Calculating Atomic Mass Example
Calculate the atomic mass of carbon.
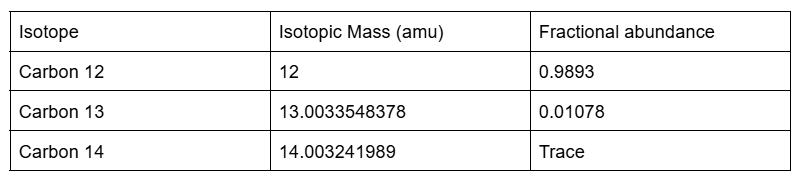
(0.9893 × 12amu) + (0.01078 × 13.0033548378) = 12.01
(Trace isotopes do not need to be considered when calculating atomic mass.)
Fractional abundance should always be calculated in decimal or fraction form NOT percentage.
Abundance via Mass Spectrometry
The occurrence and natural abundance of an isotope can be determined using an instrument called mass spectrometer.
The way it works is the spectrometer vaporizes the sample and exposes it to a high energy electron beam. This causes the sample’s atoms to become electrically charged. These cations are then separated by mass and charge.
Chemical Formulas
There are several types of chemical formulas that are used to describe a compound.
Empirical Formula: Indicates the types of atoms present as symbols and the simplest whole number ratio of the number of atoms in a compound as subscripts.
Molecular Formula: a representation of a molecule that uses chemical symbols to indicate the types of atoms followed by subscripts to show the number of atoms of each type in the molecule.
Structural Formula: Shows what atoms and number of atoms are in a molecule and also shows how the atoms are connected to each other. Multiple molecules may have the same structural formula (isomers).
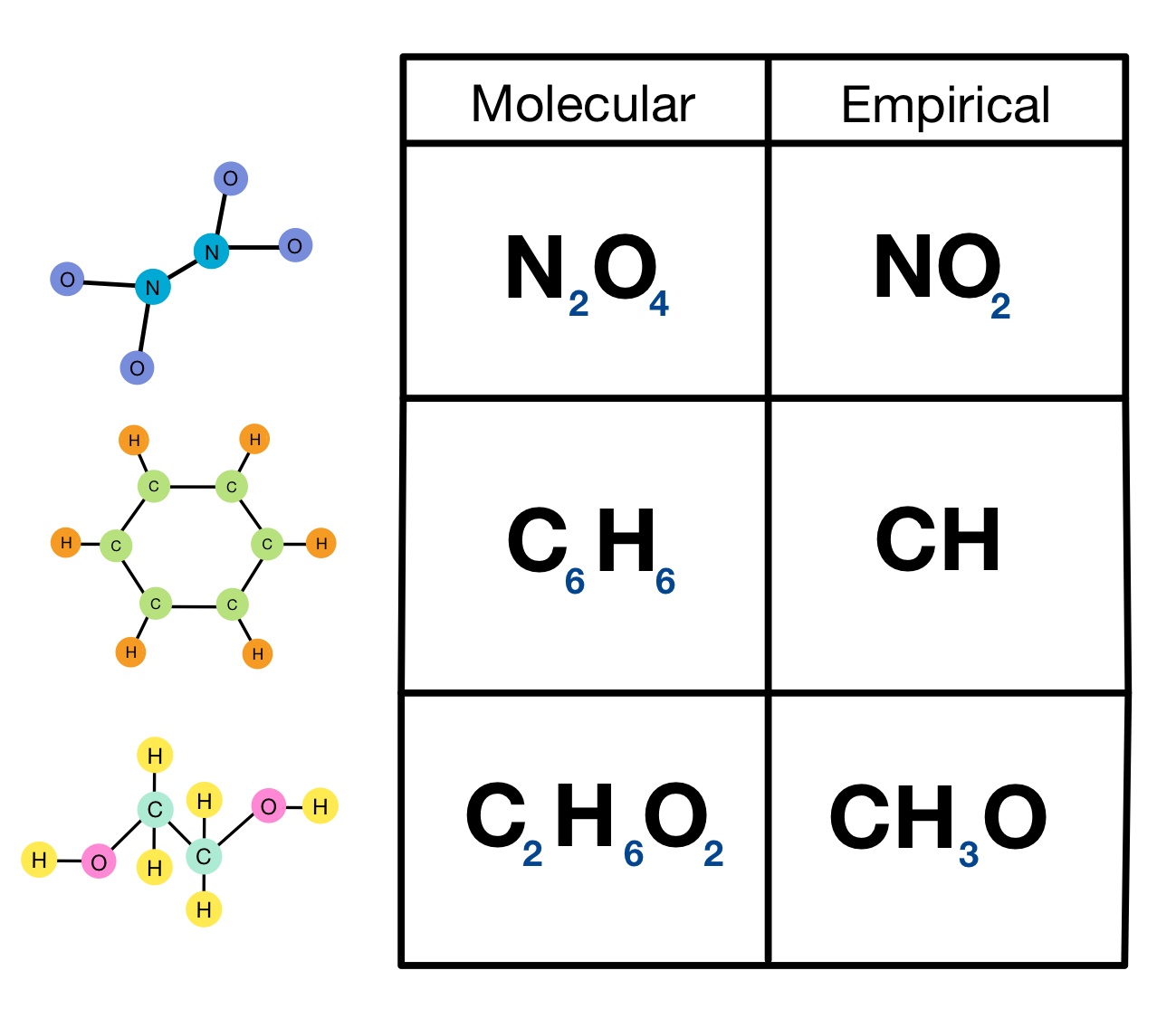
Formula Units
Remember that a molecule is composed of two or more elements that are covalently bonded.
A formula unit indicates the lowest whole number ratio of ions in an ionic compound.
A molecule will have an empirical formula and a molecular formula; however, a formula unit will only have an empirical formula.
Isomers
It is possible for the same atoms (and number of atoms) to be arranged in different ways to make different molecules with different properties.
An isomer is a compound with the same molecular formula, but a different structural formula.
Ex. Glucose, galactose, and fructose are all isomers of each other. Their molecular formulas are all C6H12O3. But they have different structural isomers and different properties.

Acetic acid and methyl formate are also isomers, same molecular formula (C2H4O2). However, they have different structures and properties.
The Mole
Mole (mol) is the chemical counting unit that allows us to connect the macroscopic and microscopic domains of chemistry.
A mole is a unit of quantity (like a dozen).
One mole = 6.0221367 × 1023 (atoms, molecules, etc.)
Importance of the Mole
The importance of the mole is that it provides a specific measure of the number of atoms, molecules, ions (anything of that microscopic level) in a bulk sample of matter so that it is easy to picturize/understand at a macroscopic level.
An atom of an element cannot be directly weighed, viewed, or handled (it is too small). But one mole of an element can.
The mole is arbitrarily defined as the amount of a substance containing the same number of discrete entities (atoms, molecules, etc.) as the number of atoms in a sample of pure carbon-12 weighting exactly 12 grams. This is 6.02214179 × 1023 (known as Avogadro’s number).
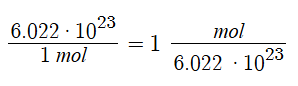
The mole provides a link between the mass of a sample and the number of discrete entities in that sample. One mole of each type of element has a unique mass.
The Mole and the Atomic Mass Unit
Both the mole and the amu are defined based on a pure sample of carbon 12.
One atom of carbon 12 has the mass of 12 amu.
This means that the masses reported on the periodic table are the masses in amu of 1 average atom of the element.
One mole of carbon 12 has the mas of 12 grams.
This means that the masses reported on the periodic table are the masses in grams of 1 mole of the element.

The atomic mass of silicon is 28.085 amu/atom.
The molar mass of silicon is 28.085g/mol.
Formula Mass vs Molar Mass
The formula mass of a compound is the sum of the atomic masses for the elements in the compound.
The molar mass of a compound is the sum of the molar masses of the elements in the compound.
Numerically the formula mass and molar mass are the same for a compound, but we will be using molar masses more frequently.
Practice Problems
Calculate the molar mass C2H6O
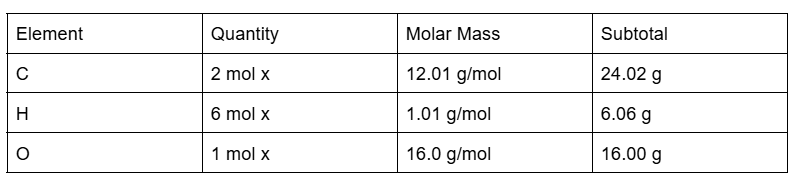
To get the total we need to add the subtotals. The total molar mass of C2H6O is 46.08g/mol.
