Quantitative Research Design
Plan for answering the research question
Quantitative Research Design provides a plan for answering the research question.
Includes plan for:
study setting
participant selection
study procedures
variable measurement
data collection
data management
data analysis
Key considerations in quantitative studies
Intervention: whether an active manipulation is applied.
Comparisons: what the control or comparison condition is.
Potential confounding variables: factors that may distort the observed effect.
Controlling study context: methods to minimize environmental or procedural variability.
Controlling participant factors:
randomization -randomly put participants in a group.
homogeneity - make sample similar
matching- match on a variable like gender, if theres a women in group a there’s on in group bThis technique ensures that both groups are comparable, thereby minimizing the effects of that variable on the study's outcomes.
statistical control
Blinding: masking participants, researchers, or assessors to group allocation when possible. In Drug Trails the Pharmacist may be unblinded in case a participant has a problem
Data collection times:
cross-sectional (Collect data all at one time) vs longitudinal designs (over a LONG period of time)
Relative timing:
prospective (forward) vs retrospective (back…example chart reviews)
Location: setting of data collection (labs, clinics, community, etc.).
Study designs (overview and evidence levels)
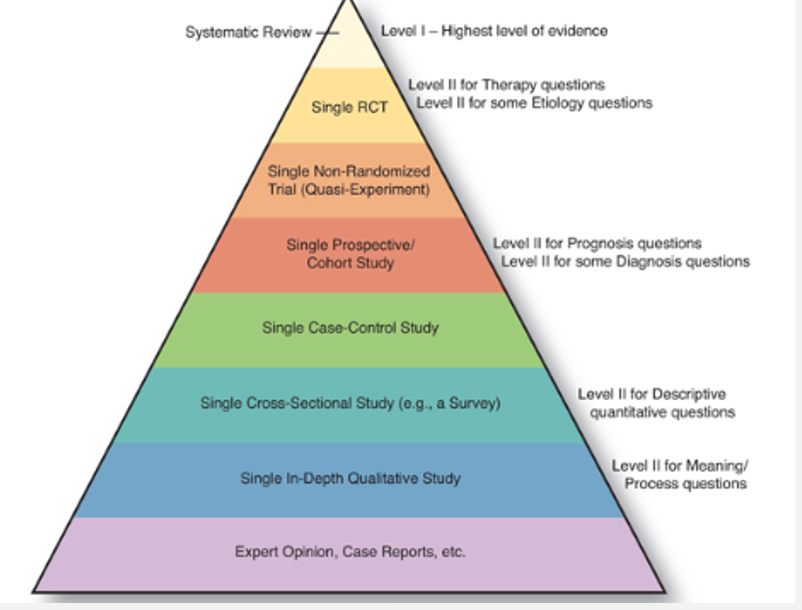
Systematic Review
Level I: Highest level of evidence
Level II for Therapy questions
Single RCT: Level II for some Etiology questions
Single Non-Randomized Trial (Quasi-Experiment)
Single Prospective Cohort Study
Level II for Prognosis questions
Level II for some Diagnosis questions
Single Case-Control Study
Single Cross-Sectional Study (e.g., a Survey)
Single In-Depth Qualitative Study
Level II for Descriptive quantitative questions
Level II for Meaning/Process questions
Expert Opinion, Case Reports, etc.
Classifications of study designs
Experimental
Quasi-experimental
Nonexperimental
Experimental designs (basic structure)
Key elements:
Intervention
Control
Randomization
Experimental group
Control group
Notation used in diagrams (conceptual):
Randomization = R, Intervention = X, Observation/Outcome = O
Examples of common designs include post-test only, pre-test–post-test, and crossover designs.
Experimental designs: common formats and notations
Post-test only design
Experimental group: R X O
Control group: R O
Pre-test – post-test design
Experimental group: R O X O
Control group: R O O
Crossover design
Structure involves sequences with periods where participants receive multiple treatments in different orders (randomized order) and cross-over between conditions.
Notation shown in slides: R O O O X O R O X O O O (illustrative representation of sequence changes across periods)
Advantages of experimental designs
Testing cause-and-effect relationships
Disadvantages of experimental designs
Balancing control with practical significance
Generalizability concerns
The need to randomize participants
Quasi-experimental designs (non-randomized manipulation)
Common structure: Intervention vs. Control without randomization
Notation reflects absence of randomization and the non-equivalent groups
Quasi-experimental designs: common types
Nonequivalent control group post-test only design
Experimental group: X O
Control group: O
Nonequivalent control group pre-test – post-test design
Experimental group: O X O O
Control group: O O O O
One group pre-test – post-test design
Group: O X O
Time series design
Multiple observations before and after an intervention: O O O X O O O
Quasi-experimental designs: advantages and disadvantages
Advantages
Practical
More acceptable to participants
Can be used when randomization is unethical
Disadvantages
More difficult to make causal inferences
Nonexperimental designs
Structure: Interventions vs. controls are not manipulated or randomized; observational in nature
Common types:
Correlation studies: examine relationships between variables that are not manipulated
Note: Correlation does NOT prove causation
Cohort designs
Case-control designs
Descriptive studies: observe, describe, and document a phenomenon
Nonexperimental designs: practical considerations
Advantages
Very practical
Efficient for collecting large amounts of data
Disadvantages
Cannot make causal inferences
Self-selection can bias results
Critiquing a quantitative study design (general approach)
Key questions to ask:
Does the design match the research question/hypothesis?
What are the strengths of the design?
What are the limitations of the design?
Validity considerations in critique
Types of validity to consider:
Statistical conclusion validity
External validity
Construct validity
Internal validity
Internal validity: threats to credibility of causal inferences
Common threats (examples):
Temporal ambiguity
Selection
History
Maturation
Mortality/Attrition
(Other common threats include instrumentation, diffusion, testing, regression to the mean, etc., though not listed explicitly in slides.)