
Chapter 1-6 Bio 81 Test
Biology: the study of life
Living organisms can either be: unicellular or multicellular
Unicellular: forms of life that consist of a single cell
Multicellular: forms of life that consist of multiple cells
How many characteristics of life? 8
What are the 8 characteristics of life? Composed of cells, have order/organization, respond to their enviornment, regulation/homeostasis, reproduction, use energy, contain genetic information, evolve and adapt
Salt concentration in blood remains relatively steady, regardless of a person's diet. This best illustrates: homeostasis
Smallest scale of life: atoms
Largest scale of life: biosphere
Rank the multiple parts of life from smallest to largest: atoms, molecules, organelles, cells, tissues, organs, organ system, species, population, community, ecosystem, biosphere
Emergent properties: when living things become more and more complex as it goes from cellular level to organ systems “the whole is greater than the sum of the parts”
At which point of the organizational hierarchy does life emerge? The cellular level
Cells: smallest unit of life
Adaption: process that enables organisms to improve survival and reproduction in their environments, this is a result of natural selection
Fitness: an organisms ability to SURVIVE and REPRODUCE
Natural selection: “survival of the fittest”
2 requirements of natural selection: (1) genetic diversity, (2) differential reproductive success where individuals leave more offspring than others
Evolution: changes in the DNA of a population over multiple generations and can occur through natural selection + responsible for life’s diversity
Evolution through natural selection will occur most rapidly for populations of plants that: reproduce sexually and live in an unstable enviornment
Taxonomy: branch of science that classifies, identifies & names organisms
Three domains of life: Bacteria, Archaea, and Eukarya
Prokaryotes: lacks a nucleus, unicellular, bacteria + archaea
Bacteria DNA: circular and found in a region called the nucleoid and have small ribosomes and divide by binary fission
Eukaryotes: uni or multicellular organisms that have a nucleus and other membrane bound organelles
Eukaryotic DNA: linear shape and found inside the nucleus has ribosomes and divides by mitosis and cytokinesis
4 Domains of Eukaryotes: animals, plants, fungi, protists
Scientific method starts with: an observation and a question
Prediction: expected outcome
Hypothesis: proposed and testable explanation for an observation that includes a prediction
Theory: testable and broad hypothesis supported by a large body of evidence
Control groups: help prevent false positives/negatives
Negative control: control group where no response is expected like placebo
Positive control: control group where a response IS expected
97% of the mass of more life is composed of: Carbon, Hydrogen, Oxygen, Nitrogen, Phosphorus, and Sulfur
Trace elements: required for life in small amounts
Shells closer to the nucleus are: lower in energy than in distant shells
Radioactive Isotopes: unstable isotopes that break down & emit energy in the form of rays or particles
Half-life: time it takes for half of all radioactive atoms in a sample to break down
Uses for Radioactive isotopes: in medicine and for radiometric dating of fossils
Intramolecular bonds: interactions between atoms within the same molecule
Intermolecular bonds: interactions between atoms of different molecules
Covalent bond: when two atoms come together to share electrons to fulfill the octet rule
Polar covalent bond: in order to reach stability, atoms share electrons to meet the illusion that each atom completes the octet rule
Hydrogen bond: when a hydrogen atom polar covalently bonded to one electronegative atom is attract to another negatively charged atom
Ionic bond: forms when metals lose and electron to the plasma field and the nonmental gains the electron and then they form a compound
Water: small polar molecule that has partial positive and negative charges
Emergent properties of water: cohesion, adhesion, surface tension, low density, high specific heat and vaporization, universal solvent
Cohesion: ability for water molecules to stick together
Adhesion: ability for water molecules to stick to other molecules that are not water
Surface tension: measure of difficulty in breaking the surface of a liquid with force
Emergent properties of water is because water is: polar and can mkae hydrogen bonds
Water adheres to: polar or charged objects
Solid ice is important because: it has lower density than liquid water and can float which insulates the liquid below and helps sustain life
Water’s specific heat: is high which allows water to resist temperature changes
Specific heat: the amount of heat required to raise or lower 1 gram of a substance 1 degree Celsius
Heat of vaporization (evaporation): amount of heat required to convert 1 gram of a liquid to a gaseous state
Water’s heat of vaporization: high heat of vaporization due to the abundance of hydrogen bonds
Universal solvent: how water is described because it can dissolve so many solutes
Solvent: substance that does the dissolving and it found in larger amounts
Solute: the substance that gets dissolved by the solvent found in smaller amounts
Solution: the combination of solute and solvent
Hydrophobic: a substance that does not have an affinity for water that is nonpolar and fears water
Hydrophilic: a substance that has an affinity/likes water and is polar and dissolves in water
What is the pH of blood: 7.4
Acids: increases a solution’s concentration of H+ ions
Bases: any chemical that decreases a solutions concentration of H+ ions
Buffers: created by combining any acid with a bicarbonate ion (HCO3-), they resist changes in pH when acids or bases are added to a solution, used to maintain homeostasis
Organic molecules: any molecules with covalently linked carbon and hydrogen atoms
Hydrocarbons: organic molecules that only contain hydrogen and carbon atoms
Properties of carbon: 4 valance electrons = 4 bonds, form covalent bonds with itself, found in all organic compounds
4 different ways carbon backbones vary: length, position of double bonds, branch points, linear vs ring
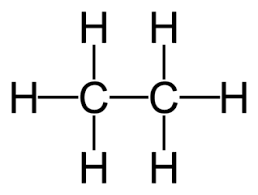
Ethane structure:
Propane structure: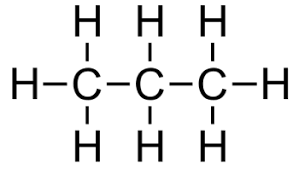
1-Butene structure: 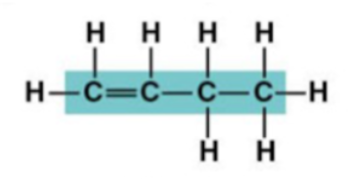
2-Butene structure: 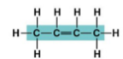
Butene Structure: 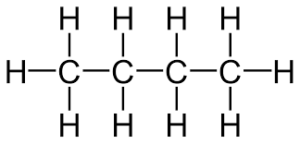 2-Methylpropane structure:
2-Methylpropane structure: 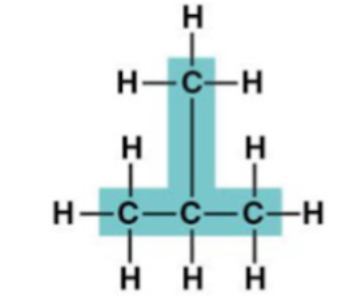
Cyclohexane structure: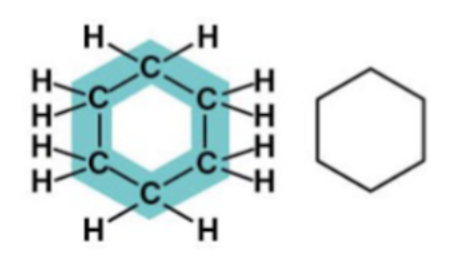
Benzene structure: 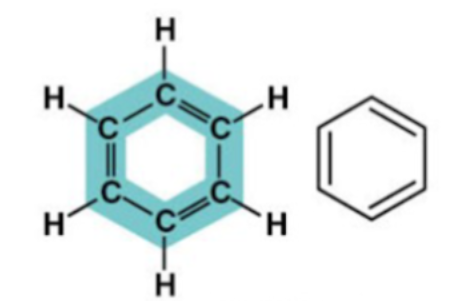 Functional groups: groups of atoms that are reactive/functional and commonly found together that typically extend off the backbone
Functional groups: groups of atoms that are reactive/functional and commonly found together that typically extend off the backbone
Hydroxyl group (alcohol): R-OH
Carboxyl group (carboxylic acid): 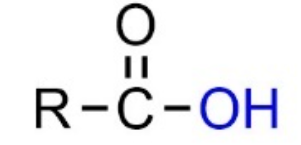
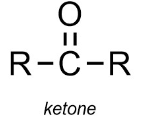
Carbonyl group (ketones/aldehydes): 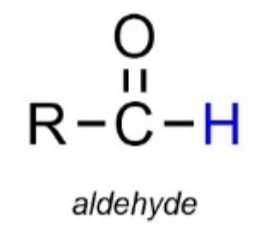
Amino groups (amine): 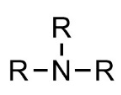
Sulfhydryl groups (thiol): 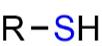
Phosphate group:
Methyl group:
Biomolecules: organic molecules that are essential to living organisms
What are the 4 biomolecules? Carbohydrates, lipids, proteins, nucleic acids
Carbohydrates: sugars and polymers of sugars that provide structural support and are used for energy storage
Lipids: does not have polymers, mix poorly with water, and consists of mostly hydrocarbon regions, and includes fats, phospholipids, and steroids
Proteins: chains of amino acids linked together by peptide bonds
Nucleic acids: DNA and RNA
Monomers: single individual building blocks that can be linked together to create polymers
Carbohydrate monomer: monosaccharide (glucose & fructose)
Glucose structure: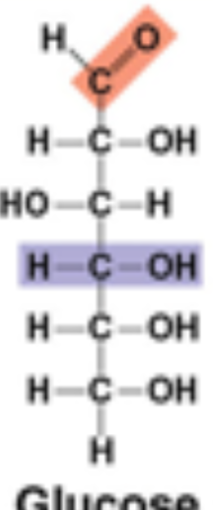
Fructose structure: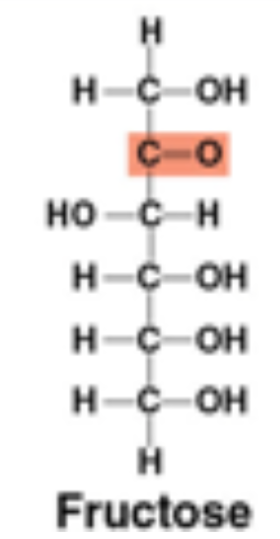
Carbohydrate polymer: disaccharides and polysaccharides
Disaccharide: formed by dehydration reaction which joins 2 monosaccharaides through a glycosidic linkage
Glycosidic bonds: covalent bonds that link monosaccharides together
Sucrose: glucose + fructose
Maltose: glucose + glucose
Polysaccharide: starch (glucose monomers), glycogen, cellulose, chitin
Glycogen: how starch is stored in our human body
Starch: how energy is stored in plant cells
Cellulose: a major component of cell wall that is linked together by covalent bonds in plants that our body cannot break down
Lipid monomer: do not have monomers or polymers
Fats: long-term energy storage in animals & plants
Phospholipids: major component of cell membranes
Steroid: component of plasma membranes and hormones
Glycerol: three carbon alcohol with a hydroxyl group attached to each carbon
Triglyceride: created by joining 3 fatty acids and joining it to a glycerol by dehydration synthesis and is needed by our cells for energy
Steroids: lipids characterized by a carbon skeleton like cholesterol and have 4 fused carbon rings
Cholesterol: precursor from which other steroids and hormones are synthesized
Saturated fatty acid: maximum number of hydrogen atoms, no double bonds, and solid at room temperature
Trans-fat: chemically altered cis-fat that turns into plaques when not turned into ATP
Unsaturated fatty acid: have one of more double bonds, has a kink, and is liquid at room temperature
Phospholipid: consists of two fatty acids and a phosphate group attached to glycerol, tails are hydrophobic while the heads are hydrophilic
Protein monomer: amino acid
Amino acid: contains a central carbon and hydrogen, amino group, carboxyl group, unique “R” group
Protein polymer: polypeptide chain
Primary: order of amino acids
Secondary: formation of alpha helices of beta sheets and are caused due to hydrogen bonding between atoms of peptide backbone
Tertiary: overall 3D shape of a polypeptide chain
Quaternary: multiple polypeptide chains to form a single functional protein
Denatured protein: a non-functional protein that has altered its shape due to changes in pH, temperature, or salt concentration
Types of amino acids: enzymatic, immune, storage, transport, hormonal, receptor, contractile, structural
4 types of proteins: non-polar, polar, acidic, basic
Nucleic acid monomer: nucleotide
Nucleic acid polymer: have 5’ & 3’ ends and store/encode genetic information
DNA vs RNA: Dna is double stranded and contains deoxyribose sugar and uses the base thymine instead of uracil
What is the difference between the sugar group in DNA and the sugar group in RNA? The sugar group in DNA has one less hydroxyl group than the sugar group in RNA.
Nucleotide: consists of ribose sugar, phosphate base, nitrogenous base
Pyrimidines: cytosine, thymine, uracil and are single ringed
Purines: guanine, adenine and are double ringed
DNA: double helix with 2 anti-parallel strands connected by hydrogen bonding
RNA: acts as a template for synthesizing proteins and forms a single nucleotide chain
Dehydration synthesis: forms covalent bonds to link monomers together and build a polymer through the loss of a water molecule
Hydrolysis: cleaves covalent bonds to break down a polymer by separating a water molecule giving one chain and H and another an OH
Ribosomes: build proteins through the process of translation and can be free in the cytoplasm or bounded to the rough ER
Protein secretion: nucleus, rough & smooth ER, Golgi apparatus, cell membrane
Cellular digestion: lysosomes, peroxisomes, vacuoles
Energy related organelles: chloroplasts, mitochondria
Cytoskeleton: microfilaments, intermediate filaments, microtubules
Cell junctions: tight junctions, gap junctions, plasmodesmata
Endomembrane system: group of membrane bound organelles inside a eukaryotic cell that has the function of secreting protein and cellular digestion
What are the 7 organelles in the endomembrane system: nucleus, endoplasmic reticulum, golgi apparatus, transport vesicles, lysosomes & peroxisomes, vacuoles, and the cell membrane
Nuclear envelope: double-membrane that surrounds the nucleus and acts as a barrier
Nuclear pores: allow entry/exit into and out of the nucleus
Nucleolus: where ribosomes are assembled
Process of making proteins (short): DNA is transcribed into RNA which is translated into a protein
Rough ER: closer to the nucleus and has a ribosome covered surface in which proteins are folded and modified
Smooth ER: synthesizes lipids and detoxifies poisons in the body
Golgi apparatus: receives vesicles and repackages contents into vesicles for export
Recycling centers for the cell: central vacuole and lysosome
Mitochondria: organelle that synthesizes lots of energy for the cell, has its own DNA, 2 membranes, and a matrix that contains the DNA
Chloroplasts: green organelles that function as the site of photosynthesis in many plant cells
ATP: adenosine triphosphate: high energy molecule used to power cellular reactions
Cellular respiration: mitochondrial process that breaks down food sources like sugars & lipids to make ATP
Endosymbiotic theory: mitochondria and chloroplasts were once independently living bacteria then the aerobic bacteria (todays mitochondria) was engulfed by the anerobic host cell and cyanobacteria (todays chloroplasts) were also engulfed and over time evolved to create todays animal and plant cell
What component of the cytoskeletons do motor proteins use to transport vesicles? Microtubules
Cilia: short structures that are used to provide cell movement
Flagella: longer structure that move like a whip to provide cell movement
Tight junctions: membrane protein that links cells creating a leakproof barrier
Desmosomes: intermediate filaments that anchor neighboring cells together
Gap junctions: protein channels that connect the cytoplasm of two animal cells in which ions can travel
Plasmodesmata: gaps in the cells walls that connect the cytoplasm of two plant cells
Chapter 1-6 Bio 81 Test
Biology: the study of life
Living organisms can either be: unicellular or multicellular
Unicellular: forms of life that consist of a single cell
Multicellular: forms of life that consist of multiple cells
How many characteristics of life? 8
What are the 8 characteristics of life? Composed of cells, have order/organization, respond to their enviornment, regulation/homeostasis, reproduction, use energy, contain genetic information, evolve and adapt
Salt concentration in blood remains relatively steady, regardless of a person's diet. This best illustrates: homeostasis
Smallest scale of life: atoms
Largest scale of life: biosphere
Rank the multiple parts of life from smallest to largest: atoms, molecules, organelles, cells, tissues, organs, organ system, species, population, community, ecosystem, biosphere
Emergent properties: when living things become more and more complex as it goes from cellular level to organ systems “the whole is greater than the sum of the parts”
At which point of the organizational hierarchy does life emerge? The cellular level
Cells: smallest unit of life
Adaption: process that enables organisms to improve survival and reproduction in their environments, this is a result of natural selection
Fitness: an organisms ability to SURVIVE and REPRODUCE
Natural selection: “survival of the fittest”
2 requirements of natural selection: (1) genetic diversity, (2) differential reproductive success where individuals leave more offspring than others
Evolution: changes in the DNA of a population over multiple generations and can occur through natural selection + responsible for life’s diversity
Evolution through natural selection will occur most rapidly for populations of plants that: reproduce sexually and live in an unstable enviornment
Taxonomy: branch of science that classifies, identifies & names organisms
Three domains of life: Bacteria, Archaea, and Eukarya
Prokaryotes: lacks a nucleus, unicellular, bacteria + archaea
Bacteria DNA: circular and found in a region called the nucleoid and have small ribosomes and divide by binary fission
Eukaryotes: uni or multicellular organisms that have a nucleus and other membrane bound organelles
Eukaryotic DNA: linear shape and found inside the nucleus has ribosomes and divides by mitosis and cytokinesis
4 Domains of Eukaryotes: animals, plants, fungi, protists
Scientific method starts with: an observation and a question
Prediction: expected outcome
Hypothesis: proposed and testable explanation for an observation that includes a prediction
Theory: testable and broad hypothesis supported by a large body of evidence
Control groups: help prevent false positives/negatives
Negative control: control group where no response is expected like placebo
Positive control: control group where a response IS expected
97% of the mass of more life is composed of: Carbon, Hydrogen, Oxygen, Nitrogen, Phosphorus, and Sulfur
Trace elements: required for life in small amounts
Shells closer to the nucleus are: lower in energy than in distant shells
Radioactive Isotopes: unstable isotopes that break down & emit energy in the form of rays or particles
Half-life: time it takes for half of all radioactive atoms in a sample to break down
Uses for Radioactive isotopes: in medicine and for radiometric dating of fossils
Intramolecular bonds: interactions between atoms within the same molecule
Intermolecular bonds: interactions between atoms of different molecules
Covalent bond: when two atoms come together to share electrons to fulfill the octet rule
Polar covalent bond: in order to reach stability, atoms share electrons to meet the illusion that each atom completes the octet rule
Hydrogen bond: when a hydrogen atom polar covalently bonded to one electronegative atom is attract to another negatively charged atom
Ionic bond: forms when metals lose and electron to the plasma field and the nonmental gains the electron and then they form a compound
Water: small polar molecule that has partial positive and negative charges
Emergent properties of water: cohesion, adhesion, surface tension, low density, high specific heat and vaporization, universal solvent
Cohesion: ability for water molecules to stick together
Adhesion: ability for water molecules to stick to other molecules that are not water
Surface tension: measure of difficulty in breaking the surface of a liquid with force
Emergent properties of water is because water is: polar and can mkae hydrogen bonds
Water adheres to: polar or charged objects
Solid ice is important because: it has lower density than liquid water and can float which insulates the liquid below and helps sustain life
Water’s specific heat: is high which allows water to resist temperature changes
Specific heat: the amount of heat required to raise or lower 1 gram of a substance 1 degree Celsius
Heat of vaporization (evaporation): amount of heat required to convert 1 gram of a liquid to a gaseous state
Water’s heat of vaporization: high heat of vaporization due to the abundance of hydrogen bonds
Universal solvent: how water is described because it can dissolve so many solutes
Solvent: substance that does the dissolving and it found in larger amounts
Solute: the substance that gets dissolved by the solvent found in smaller amounts
Solution: the combination of solute and solvent
Hydrophobic: a substance that does not have an affinity for water that is nonpolar and fears water
Hydrophilic: a substance that has an affinity/likes water and is polar and dissolves in water
What is the pH of blood: 7.4
Acids: increases a solution’s concentration of H+ ions
Bases: any chemical that decreases a solutions concentration of H+ ions
Buffers: created by combining any acid with a bicarbonate ion (HCO3-), they resist changes in pH when acids or bases are added to a solution, used to maintain homeostasis
Organic molecules: any molecules with covalently linked carbon and hydrogen atoms
Hydrocarbons: organic molecules that only contain hydrogen and carbon atoms
Properties of carbon: 4 valance electrons = 4 bonds, form covalent bonds with itself, found in all organic compounds
4 different ways carbon backbones vary: length, position of double bonds, branch points, linear vs ring
Ethane structure:

Propane structure:
1-Butene structure: 
2-Butene structure: 
Butene Structure:  2-Methylpropane structure:
2-Methylpropane structure: 
Cyclohexane structure:
Benzene structure:  Functional groups: groups of atoms that are reactive/functional and commonly found together that typically extend off the backbone
Functional groups: groups of atoms that are reactive/functional and commonly found together that typically extend off the backbone
Hydroxyl group (alcohol): R-OH
Carboxyl group (carboxylic acid): 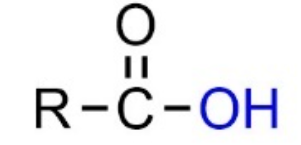
Carbonyl group (ketones/aldehydes): 

Amino groups (amine): 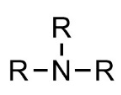
Sulfhydryl groups (thiol): 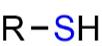
Phosphate group:
Methyl group:
Biomolecules: organic molecules that are essential to living organisms
What are the 4 biomolecules? Carbohydrates, lipids, proteins, nucleic acids
Carbohydrates: sugars and polymers of sugars that provide structural support and are used for energy storage
Lipids: does not have polymers, mix poorly with water, and consists of mostly hydrocarbon regions, and includes fats, phospholipids, and steroids
Proteins: chains of amino acids linked together by peptide bonds
Nucleic acids: DNA and RNA
Monomers: single individual building blocks that can be linked together to create polymers
Carbohydrate monomer: monosaccharide (glucose & fructose)
Glucose structure:
Fructose structure:
Carbohydrate polymer: disaccharides and polysaccharides
Disaccharide: formed by dehydration reaction which joins 2 monosaccharaides through a glycosidic linkage
Glycosidic bonds: covalent bonds that link monosaccharides together
Sucrose: glucose + fructose
Maltose: glucose + glucose
Polysaccharide: starch (glucose monomers), glycogen, cellulose, chitin
Glycogen: how starch is stored in our human body
Starch: how energy is stored in plant cells
Cellulose: a major component of cell wall that is linked together by covalent bonds in plants that our body cannot break down
Lipid monomer: do not have monomers or polymers
Fats: long-term energy storage in animals & plants
Phospholipids: major component of cell membranes
Steroid: component of plasma membranes and hormones
Glycerol: three carbon alcohol with a hydroxyl group attached to each carbon
Triglyceride: created by joining 3 fatty acids and joining it to a glycerol by dehydration synthesis and is needed by our cells for energy
Steroids: lipids characterized by a carbon skeleton like cholesterol and have 4 fused carbon rings
Cholesterol: precursor from which other steroids and hormones are synthesized
Saturated fatty acid: maximum number of hydrogen atoms, no double bonds, and solid at room temperature
Trans-fat: chemically altered cis-fat that turns into plaques when not turned into ATP
Unsaturated fatty acid: have one of more double bonds, has a kink, and is liquid at room temperature
Phospholipid: consists of two fatty acids and a phosphate group attached to glycerol, tails are hydrophobic while the heads are hydrophilic
Protein monomer: amino acid
Amino acid: contains a central carbon and hydrogen, amino group, carboxyl group, unique “R” group
Protein polymer: polypeptide chain
Primary: order of amino acids
Secondary: formation of alpha helices of beta sheets and are caused due to hydrogen bonding between atoms of peptide backbone
Tertiary: overall 3D shape of a polypeptide chain
Quaternary: multiple polypeptide chains to form a single functional protein
Denatured protein: a non-functional protein that has altered its shape due to changes in pH, temperature, or salt concentration
Types of amino acids: enzymatic, immune, storage, transport, hormonal, receptor, contractile, structural
4 types of proteins: non-polar, polar, acidic, basic
Nucleic acid monomer: nucleotide
Nucleic acid polymer: have 5’ & 3’ ends and store/encode genetic information
DNA vs RNA: Dna is double stranded and contains deoxyribose sugar and uses the base thymine instead of uracil
What is the difference between the sugar group in DNA and the sugar group in RNA? The sugar group in DNA has one less hydroxyl group than the sugar group in RNA.
Nucleotide: consists of ribose sugar, phosphate base, nitrogenous base
Pyrimidines: cytosine, thymine, uracil and are single ringed
Purines: guanine, adenine and are double ringed
DNA: double helix with 2 anti-parallel strands connected by hydrogen bonding
RNA: acts as a template for synthesizing proteins and forms a single nucleotide chain
Dehydration synthesis: forms covalent bonds to link monomers together and build a polymer through the loss of a water molecule
Hydrolysis: cleaves covalent bonds to break down a polymer by separating a water molecule giving one chain and H and another an OH
Ribosomes: build proteins through the process of translation and can be free in the cytoplasm or bounded to the rough ER
Protein secretion: nucleus, rough & smooth ER, Golgi apparatus, cell membrane
Cellular digestion: lysosomes, peroxisomes, vacuoles
Energy related organelles: chloroplasts, mitochondria
Cytoskeleton: microfilaments, intermediate filaments, microtubules
Cell junctions: tight junctions, gap junctions, plasmodesmata
Endomembrane system: group of membrane bound organelles inside a eukaryotic cell that has the function of secreting protein and cellular digestion
What are the 7 organelles in the endomembrane system: nucleus, endoplasmic reticulum, golgi apparatus, transport vesicles, lysosomes & peroxisomes, vacuoles, and the cell membrane
Nuclear envelope: double-membrane that surrounds the nucleus and acts as a barrier
Nuclear pores: allow entry/exit into and out of the nucleus
Nucleolus: where ribosomes are assembled
Process of making proteins (short): DNA is transcribed into RNA which is translated into a protein
Rough ER: closer to the nucleus and has a ribosome covered surface in which proteins are folded and modified
Smooth ER: synthesizes lipids and detoxifies poisons in the body
Golgi apparatus: receives vesicles and repackages contents into vesicles for export
Recycling centers for the cell: central vacuole and lysosome
Mitochondria: organelle that synthesizes lots of energy for the cell, has its own DNA, 2 membranes, and a matrix that contains the DNA
Chloroplasts: green organelles that function as the site of photosynthesis in many plant cells
ATP: adenosine triphosphate: high energy molecule used to power cellular reactions
Cellular respiration: mitochondrial process that breaks down food sources like sugars & lipids to make ATP
Endosymbiotic theory: mitochondria and chloroplasts were once independently living bacteria then the aerobic bacteria (todays mitochondria) was engulfed by the anerobic host cell and cyanobacteria (todays chloroplasts) were also engulfed and over time evolved to create todays animal and plant cell
What component of the cytoskeletons do motor proteins use to transport vesicles? Microtubules
Cilia: short structures that are used to provide cell movement
Flagella: longer structure that move like a whip to provide cell movement
Tight junctions: membrane protein that links cells creating a leakproof barrier
Desmosomes: intermediate filaments that anchor neighboring cells together
Gap junctions: protein channels that connect the cytoplasm of two animal cells in which ions can travel
Plasmodesmata: gaps in the cells walls that connect the cytoplasm of two plant cells
 Knowt
Knowt