Advances in molecular diagnosis of TB
Advances in Molecular Diagnosis of Tuberculosis
Authors and Affiliations
Emily MacLean (Department of Epidemiology, Biostatistics, and Occupational Health, McGill University)
Mikashmi Kohli (McGill International TB Centre, McGill University)
Stefan F. Weber (Department of Infectious Diseases, University of Heidelberg)
Anita Suresh (Foundation for Innovative New Diagnostics, Geneva, Switzerland)
Samuel G. Schumacher (Foundation for Innovative New Diagnostics)
Claudia M. Denkinger (Departments of Infectious Diseases and McGill International TB Centre)
Madhukar Pai (McGill International TB Centre; Manipal McGill Program for Infectious Diseases, Manipal Academy of Higher Education)
Abstract
Molecular tests for tuberculosis (TB) are essential tools designed to identify approximately 3 million undiagnosed or unreported TB cases annually, ensuring timely treatment and reducing transmission rates. These tests offer rapid and precise results, which include crucial drug-susceptibility testing that is vital for managing TB effectively. The World Health Organization (WHO) recommends molecular nucleic acid amplification tests (NAATs) over traditional smear microscopy due to their superior detection accuracy, particularly in patients with paucibacillary disease or co-infection with HIV. Several molecular tests have also advanced to pinpoint mycobacterial drug resistance mutations, which aids in tailoring treatment regimens to individual patient needs. Continuous innovations in molecular diagnostics strive to enhance accessibility in both reference laboratories and peripheral health settings, especially in countries heavily burdened by TB.
Background
In 2018, TB was responsible for approximately 1.5 million deaths and 10 million new infections globally, establishing itself as the leading infectious disease killer. The WHO's End TB Strategy aims to identify the 3 million missing TB patients to achieve the objective of eliminating TB as a public health threat by 2030. Novel diagnostic advances are critical to achieving this goal, particularly considering the disruptive challenges presented by the COVID-19 pandemic, which has affected healthcare delivery systems worldwide.
Molecular Diagnosis Advancements
The past decade has witnessed significant advancements through NAATs, especially PCR-based techniques, which have transformed TB diagnostics by dramatically improving the speed and accuracy of detection. These molecular techniques facilitate immediate results for drug susceptibility testing against key first-line medications such as rifampicin (RIF) and isoniazid (INH), critical for effective treatment management. Despite the promising developments in TB diagnostics, global implementation remains hindered by various logistical barriers, including discrepancies in healthcare infrastructure, training, and resource allocation across different nations.
State of the Art
Currently, numerous WHO-endorsed molecular TB tests are available, including the Xpert MTB/RIF assay, endorsed in 2010. Recent innovations continue to emerge, guided by specified product profiles to suit diverse healthcare environments. Identifying and addressing the challenges inherent in the adoption of these advanced technologies is vital to scaling their application in settings that bear the highest disease burden.
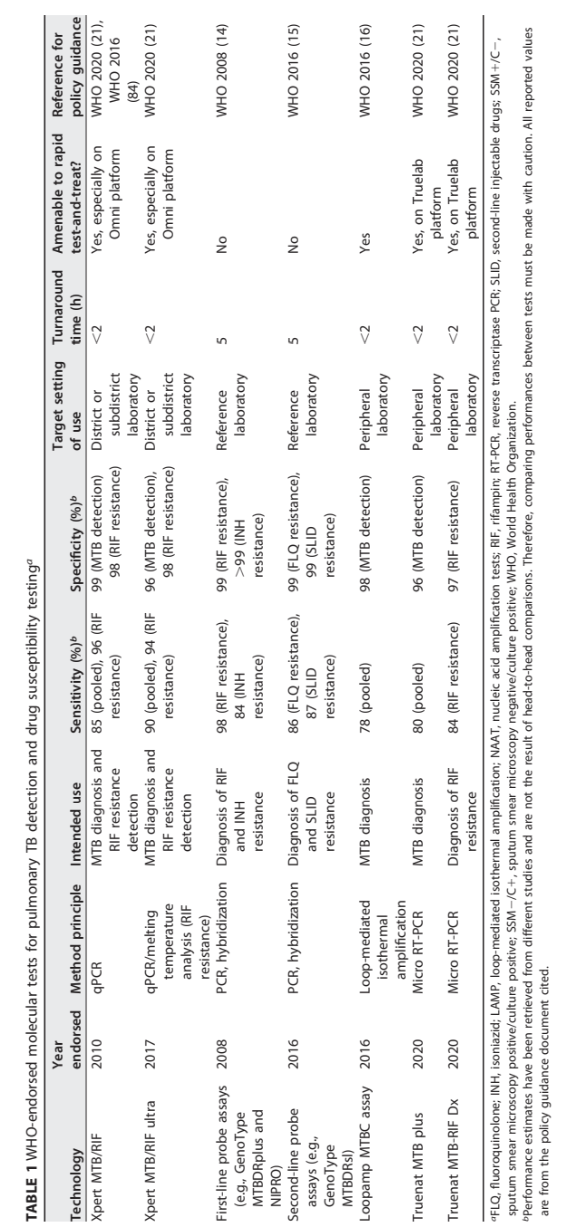
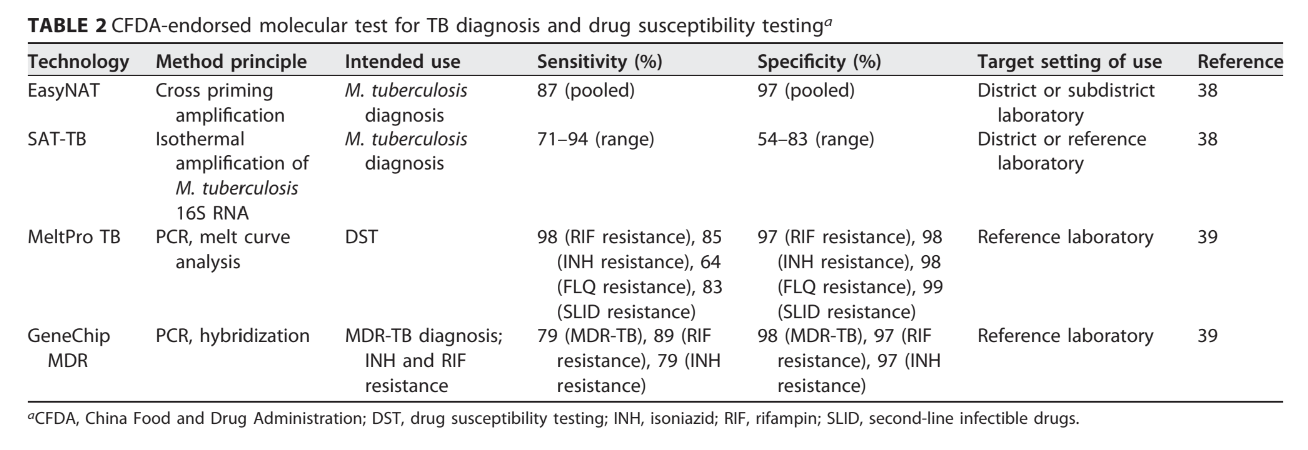
WHO-Endorsed Molecular Tests
Overview of Available Tests
(See Table 1)
Xpert MTB/RIF (2010): Features an 85% sensitivity and 99% specificity; extensively utilized across district laboratories worldwide.
Xpert MTB/RIF Ultra (2017): Demonstrates improved sensitivity (90%) for both diagnosis and resistance detection, enhancing its utility.
First-line probe assays (e.g., GenoType MTBDRplus): Targets resistance to RIF and INH; noted for high sensitivity in specialized reference laboratories.
Loopamp MTBC assay (2016): Designed for use in peripheral laboratories with a 78% sensitivity for TB, promoting accessibility in underserved regions.
Limitations and Barriers
Despite the high accuracy and rapid results offered by contemporary molecular tests, traditional smear microscopy continues to dominate the diagnostic landscape in many regions, with a testing ratio as high as 6:1 in some areas. Overcoming operational barriers, including training of personnel and procurement of necessary equipment, is essential for maximizing the effectiveness of these advanced diagnostic tests in high TB burden countries.
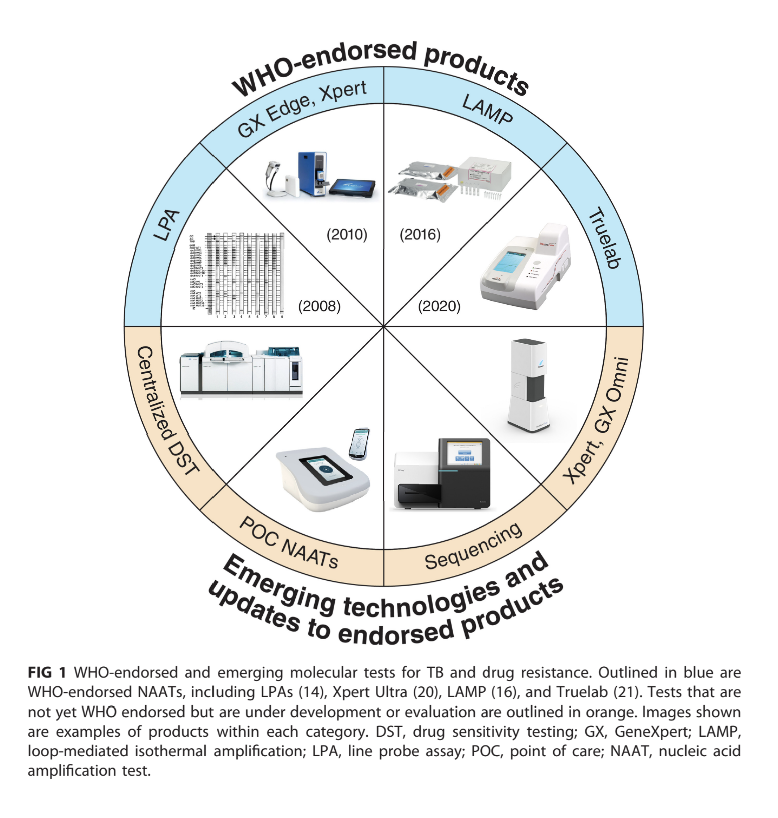
Developments in Molecular Platforms
Types of Tests and Their Applications
Line Probe Assays (LPA): Designed for drug resistance detection; ongoing enhancements lead to higher sensitivity versions capable of detecting multiple resistance patterns simultaneously.
GeneXpert Ultra: A highly anticipated new test for TB diagnostics that provides quicker results in a user-friendly design but necessitates robust infrastructural support for implementation.
Truenat and Truelab: Cost-effective micro real-time PCR assays that are specifically designed for ease of use in regions heavily affected by TB.
Emerging Technologies
Xpert XDR: On the horizon, this test aims to enhance detection capabilities for extensive drug resistance traits in TB strains, significantly expanding the diagnostic toolkit.
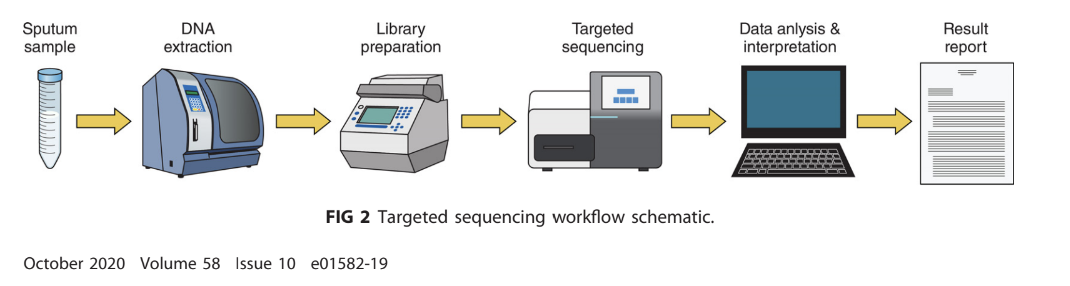
Next-Generation Sequencing (NGS): Holds promise for comprehensive drug susceptibility testing due to its capability for rapid results compared to traditional methodologies.
Integration of TB and COVID-19 Testing
The COVID-19 pandemic presents a unique opportunity to merge TB testing with COVID-19 diagnostics, thereby optimizing patient management and reducing the need for multiple follow-up visits. Countries are urged to utilize existing NAAT platforms to allow for simultaneous testing of both diseases, which can improve overall treatment outcomes.
Conclusion
The advances in molecular TB diagnostics have catalyzed significant improvements in both detection speed and accuracy. However, for these advancements to truly impact TB control efforts globally, healthcare systems must bolster their infrastructure and ensure the seamless integration of innovative testing technologies into routine healthcare practices.
Acknowledgments
This section details the contributions made towards the manuscript's preparation and acknowledges the collaborative efforts of the affiliated organizations.
References
Citations encompass key studies, WHO documents, and pertinent literature centered on TB diagnostics and treatment innovations, reinforcing the evidence base for the discussed advancements.