Lipids
Lipids are a diverse group of non-polar molecules that contain carbon, hydrogen and oxygen. Lipids include fatty acids, triglycerides, phospholipids and steroids. Lipids will only dissolve in non-polar solvents, such as alcohol, but not in water (a polar solvent).
Fatty acids
Fatty acids are composed of a carboxylic acid with a long, non-polar hydrocarbon tail. They can be saturated or unsaturated. Saturated fatty acids only have single C-C bonds in the hydrocarbon tail giving a straight tail. Unsaturated fatty acids have at least on double or triple C-C bond in the hydrocarbon tail giving a bent tail.
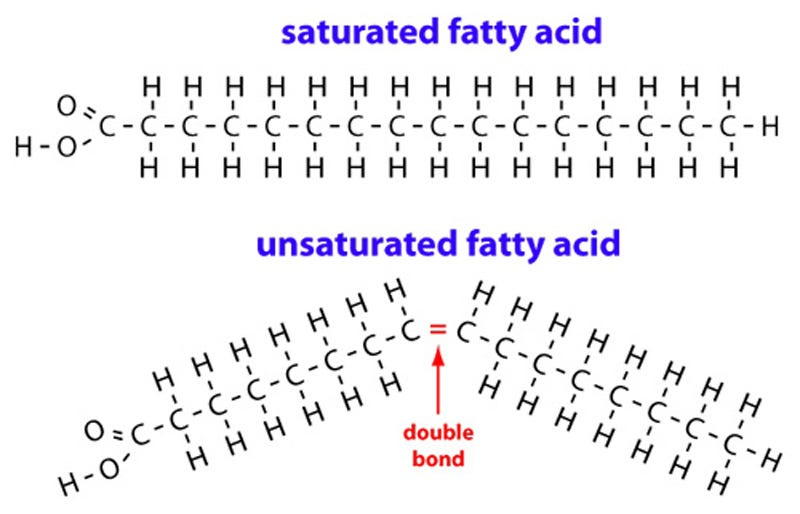
Essential fatty acids are those that must be included in the diet since they cannot be synthesised by the body.
Triglycerides
Triglycerides are formed in a condensation reaction between glycerol and 3 fatty acids (may be the same or different). The bond between a glycerol and a fatty acid is called an ester bond. This means that forming one molecule of a triglyceride releases 3 water molecules and forms 3 ester bonds.
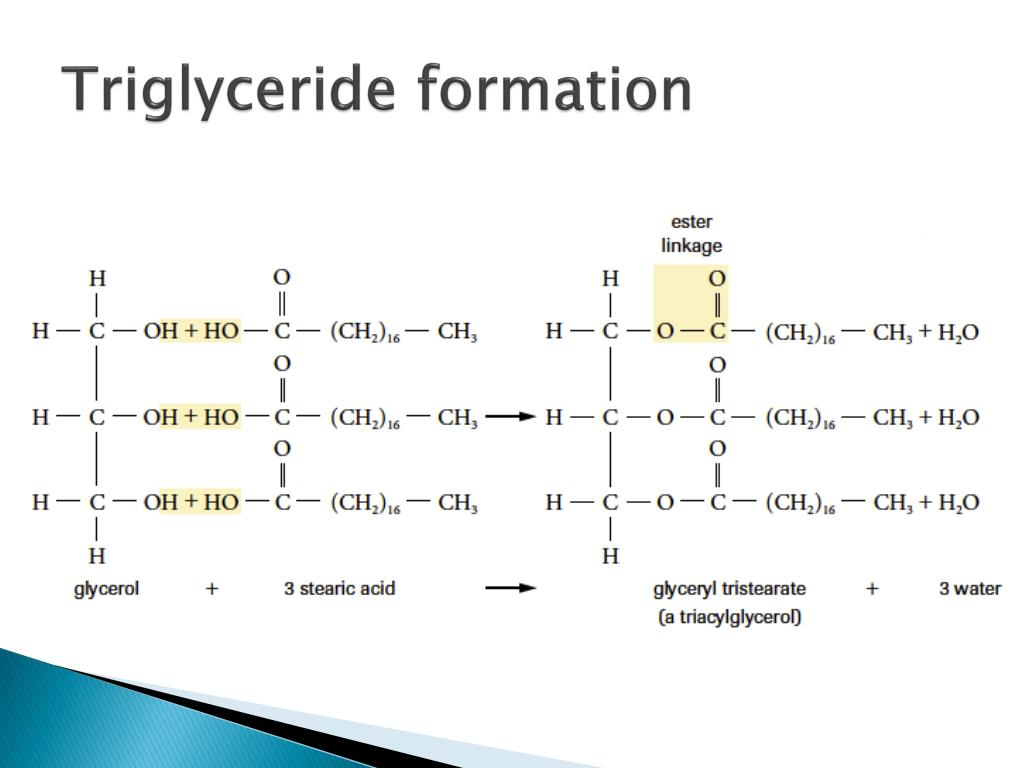
Animal store triglycerides as fat while plants store triglycerides as oil. In fats, most of the fatty acids are saturated meaning the triglyceride is solid at room temperature (as the triglycerides can pack more closely to each other). In oils, most of the fatty acids are unsaturated meaning the triglyceride is liquid at room temperature.
Roles of triglycerides:
Energy store: Important, high energy stores and are insoluble so can be stored in cells. They are found in the adipose tissue in mammals and in seeds and fruits in plants.
Thermal insulation: Insulating layer beneath the skin reduces heat loss.
Buoyancy: Fats are less dense than water e.g. blubber in whales.
Shock absorber: Deposited around vital organs to protect the from impact.
Triglycerides are important energy stores. They can be hydrolysed to 3 fatty acids and glycerol by enzymes called lipases. The fatty acids contain many C-H bonds that can be broken to release energy to produce ATP. The water produced in aerobic respiration is an important source of metabolic water.
Triglycerides and most other lipids must be transported in the blood as lipoproteins as they are non-polar and insoluble in water so they are complexed with proteins that are soluble for transport in the plasma.
Phospholipids
Phospholipids are composed of a glycerol bonded to 2 fatty acids by ester bonds and a phosphate group.
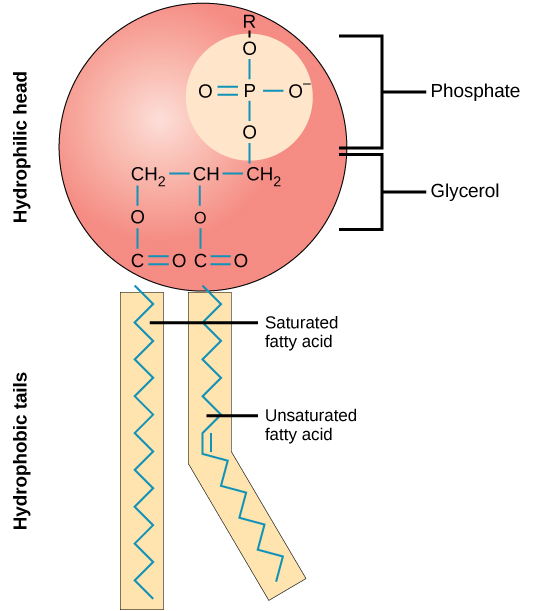
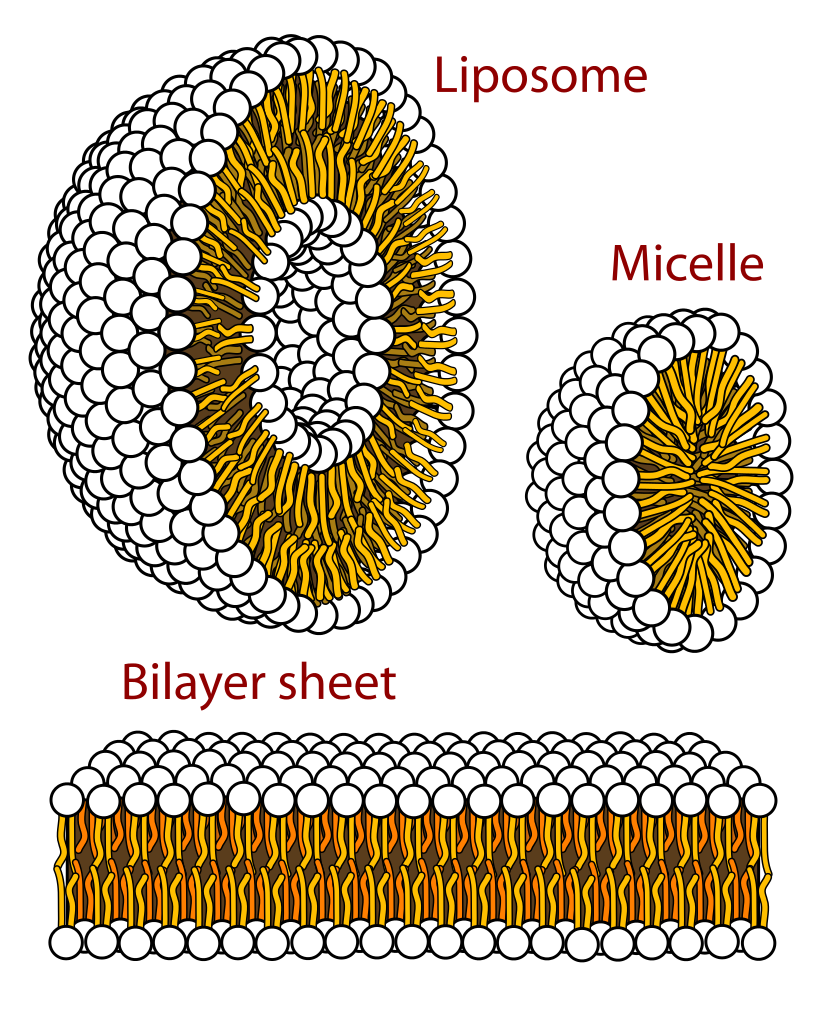
Phospholipids are split into a hydrophilic region which is the polar, phosphate-containing head and a hydrophobic region which is the non-polar fatty acid tails. This means that in water, phospholipids arrange themselves in 3 different ways: a liposome, micelle, or bilayer. In all these structures, the polar heads interact with water to face outwards, while the non-polar tails interact with each other to face inwards or towards each other to exclude water. The phospholipid bilayer forms the structural basis of all biological membranes.
Some phospholipids may be modified by the addition of a small organic molecule (head group) to the phosphate group such as choline (to form phosphatidylcholine) or serine (to form phosphatidylserine). In glycolipids, the phosphate group is replaced with a carbohydrate chain. These modifications give the phospholipids and glycolipids different roles within membranes.
Steroids
Cholesterol is a small non-polar molecule with 4 carbon-based rings (isoprene units) and a single polar OH group at one end. Cholesterol is an important component of membranes and fits in-between the phospholipids. It helps regulate the fluidity of membranes by preventing it from becoming too fluid or too solid. Cholesterol is also used in the synthesis of the steroid hormones e.g. testosterone, and oestrogen

Excess cholesterol in the blood is a problem in humans as it may be deposited in the walls of blood vessels leading to atherosclerosis (narrowing of artery). Also, excess cholesterol in bile may lead to gall stones in the gall bladder.
Receptors that bind to steroid hormones are usually found in the cytoplasm as they are small and hydrophobic so can easily pass through the hydrophobic part of the plasma membrane.