
Chapter 3 & 4 Notes
Transition Metals
3.1
Electronic Configurations
- d-block elements formed as the 3d, 4d, 5d are filled with e-
- Cr: [Ar]3d54s1 & Cu: [Ar]3d104s1
- 2nd row & 3rd row configurations aren’t obvious due to shielding effects and pairing electrons
Oxidation States
- the maximum oxidation states increase with group number, +3 for group 3, +7 for group 7
- further towards the end of a series it is difficult to use 4s & 3d e- in bonding
- ex. Zinc is almost never used in bonding bc 3d e- are core e-
- when an element is in a lower state than the group #, it may possess unpaired d electrons, results in magnetic & optical properties
Ligands & Complexes
- Transition-Metal Complexes: a transition metal atom bonded to several ions or molecules
- if it carries a charge it is called a complex ion or ionic complex
- ligand: molecule/ion bonded directly to a transition metal (can be neutral or anions)
- coordinated anions always end in the letter “o”
- most metal-ligand bonds are polar covalent bonds, lone pairs of electrons are shared equally between the metal and the ligand
- Notation: square brackets ex. [Co(NH3)6]3+
- Cation is always indicated first, with ligand in the square brackets
- Counter-ions are not part of the complex: [Co(NH3)6]Cl3
- To find the charge of the metal ion:
- X + (charge of anion) = charge of complex, solve for x
Categories of Ligands
- Monodentate (one tooth): only one donor atom
- Bidentate ligand: 2 donor atoms
- Chelate ligand: 2/more donor atoms of the same ligand bound to the same metal centre, particularly strong bonding (chelate means claw)
- Bridging or terminal ligand
Isomerism
- Different spatial arrangement, same molecular formula, different connectivity
- Linkage Isomers: complexes differ by only the donor atom (type of constitutional isomer)
- when naming donor atom is in italics after the ligand name
- if the ligands are neutral, the charge is the same as the oxidation state of the metal, if ligands are anionic, complex may be neutral or negatively charged
- Coordination Isomers: combining complex cations and complex anions to make salts, which differ only by which metal is in the cation or anion
- Ligand interchange between the 2 metal centres
- Ionization Isomer: type of constitutional isomer, results from an interchange of an anionic ligand with in coordination sphere with anion outside
Coordination Numbers & Stereochemistry
- Structure of the complex is defined by…
- Coordination Number: # of atoms directly bound to the metal centre (# of teeth)
- Stereochemistry: describes how coordinated atoms are arranged in space
- Coordination # 2 is linear
- Coordination # 3 is trigonal planar
- CN 2&3 are uncommon for transition metal ions except Cu(I), Ag(I), Au(I)
- Coordination # 4 is tetrahedral or square planar
- to make neutral complexes from Co(II)/Pt(II), 2 neutral ligands & 2 anionic ligands are req’d
- [CoX2L2] only has 1 possible structure (tetrahedral) because of bond angles
- [PtX2L2] can have 2 arrangements, either X-X 90º/180º
- Cis isomer: same side (90º), Trans isomer: opposite (180º)
 Geometric isomers are not superimposable, different isomers have different functions
Geometric isomers are not superimposable, different isomers have different functions- Coordination # 5 is trigonal bipyramidal or square pyramidal
- Coordination # 6 is octahedral
- If arrangement is [MA2B4]
- Cis or trans for the A ligand
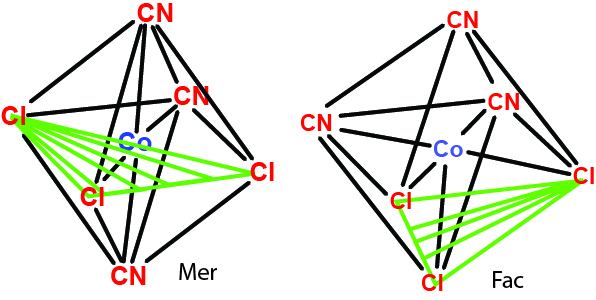
- If arrangement is [MA3B3]
- Facial (fae) isomer: 3 of the same ligands lie on the same face
- Meridian (mer) isomer: 2 are trans & 1 is cis to these
- If arrangement is [MA2B4]
![]() Carbonyl Complexes
Carbonyl Complexes
- Ligands are CO molecules (carbonyl group) (liquid @ room temp., volatile)
Porphyrins
- Type of ligand, the metal atom bonds to the middle (Fe2+), basic structure
has a charge of -2 - N donor atoms coordinate to the metal center, 2 more ligands can bind to
above and below to form octahedral geometry
Hemoglobin
- Transports oxygen from the lungs to the rest of the body
- Tetrahedral shape
- Subunits composed of a protein (globin) linked to a heme (Fe2+ porphyrin) group in a square planar configuration
- The 5th coordination is a histidine side chain (amino acid globin) which produces O2 when dissolved
- The 6th coordination can be O2
- Hemoglobin is made up of 4 polypeptide chains (4 heme groups), each heme group has 1 Fe2+ atom
- Uses Le Châtelier’s principle to release the O2 in deoxygenated areas
- inefficient, large molecule to carry small O2 molecule
- CO is an competitor to O2, the brain becomes oxygen deprived
3.2
Crystal Field Theory: electrostatic field of the ligands
Assumptions:
- Transition metal ion is treated as a free ion (not bounded to ligands)
- The e- are treated as point charges
- Ligands are negatively charged
Orbital Energies (d Orbital Splitting)
- For a free metal ion, all d orbitals are degenerate (same energies)
- If the metal is part of a octahedral complex, the energy levels are not the same as free metal ion because the environment is different
- In octahedral complexes is not spherically symmetrical, therefore the 6 point charges are in an octahedral arrangement along the metal-ligand bonding directions
- dxy, dxz, dyz (point between the ligands) are degenerate, called t2g orbitals (lower energy)
- dz2, dx2-y2 (point directly at ligands) are degenerate, called eg orbitals (higher energy)
- the energy separation between eg and t2g orbitals is Δo (‘o’ refers to octahedral)
- total energy is unchanged from barycenter (energy in spherical field) “if something goes up, something must go down”
Factors Affecting Δo
- Oxidation State of Metal Ion: increases with increasing charge of the metal
 Identity of the Metal: increases going down a group
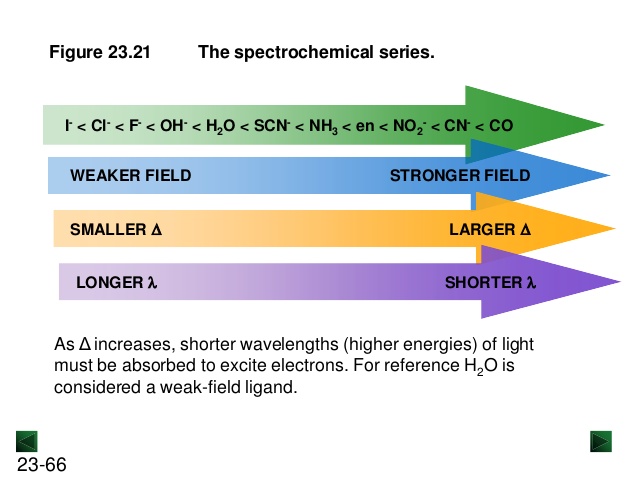
Identity of the Metal: increases going down a group- Nature of the ligand: increases along
spectrochemical series - Geometry of the complex: the d orbital
splitting pattern of an octahedral
complex is different from that of a
tetrahedral/square planar complex
![]() Colours of Transition Metal Complexes
Colours of Transition Metal Complexes
- Our eyes see the colour complementary to the colour that is absorbed
- RGB Colour Model
- Light in the visible spectrum excites e- from a t2g to eg orbital
v is frequency
Absorption Spectroscopy (UV-visible)
- Colours and colour intensities absorbed can be measured by passing light through it
Magnetism in Transition Metal Complexes
- more unpaired electrons = more paramagnetism
- High-Spin Configuration: fill all orbitals before doubling up
- Low-Spin Configuration: fill all the lower energy orbitals before the higher energy levels
- If Δo is small it doesn’t require that much energy to get into a higher energy level, therefore e- would rather jump up than pair up (pairing requires more energy
- Pairing energy: required to overcome the repulsion between 2 e- occupying the same orbital
- More stable when least repulsion
Chapter 3 & 4 Notes
Transition Metals
3.1
Electronic Configurations
- d-block elements formed as the 3d, 4d, 5d are filled with e-
- Cr: [Ar]3d54s1 & Cu: [Ar]3d104s1
- 2nd row & 3rd row configurations aren’t obvious due to shielding effects and pairing electrons
Oxidation States
- the maximum oxidation states increase with group number, +3 for group 3, +7 for group 7
- further towards the end of a series it is difficult to use 4s & 3d e- in bonding
- ex. Zinc is almost never used in bonding bc 3d e- are core e-
- when an element is in a lower state than the group #, it may possess unpaired d electrons, results in magnetic & optical properties
Ligands & Complexes
- Transition-Metal Complexes: a transition metal atom bonded to several ions or molecules
- if it carries a charge it is called a complex ion or ionic complex
- ligand: molecule/ion bonded directly to a transition metal (can be neutral or anions)
- coordinated anions always end in the letter “o”
- most metal-ligand bonds are polar covalent bonds, lone pairs of electrons are shared equally between the metal and the ligand
- Notation: square brackets ex. [Co(NH3)6]3+
- Cation is always indicated first, with ligand in the square brackets
- Counter-ions are not part of the complex: [Co(NH3)6]Cl3
- To find the charge of the metal ion:
- X + (charge of anion) = charge of complex, solve for x
Categories of Ligands
- Monodentate (one tooth): only one donor atom
- Bidentate ligand: 2 donor atoms
- Chelate ligand: 2/more donor atoms of the same ligand bound to the same metal centre, particularly strong bonding (chelate means claw)
- Bridging or terminal ligand
Isomerism
- Different spatial arrangement, same molecular formula, different connectivity
- Linkage Isomers: complexes differ by only the donor atom (type of constitutional isomer)
- when naming donor atom is in italics after the ligand name
- if the ligands are neutral, the charge is the same as the oxidation state of the metal, if ligands are anionic, complex may be neutral or negatively charged
- Coordination Isomers: combining complex cations and complex anions to make salts, which differ only by which metal is in the cation or anion
- Ligand interchange between the 2 metal centres
- Ionization Isomer: type of constitutional isomer, results from an interchange of an anionic ligand with in coordination sphere with anion outside
Coordination Numbers & Stereochemistry
- Structure of the complex is defined by…
- Coordination Number: # of atoms directly bound to the metal centre (# of teeth)
- Stereochemistry: describes how coordinated atoms are arranged in space
- Coordination # 2 is linear
- Coordination # 3 is trigonal planar
- CN 2&3 are uncommon for transition metal ions except Cu(I), Ag(I), Au(I)
- Coordination # 4 is tetrahedral or square planar
- to make neutral complexes from Co(II)/Pt(II), 2 neutral ligands & 2 anionic ligands are req’d
- [CoX2L2] only has 1 possible structure (tetrahedral) because of bond angles
- [PtX2L2] can have 2 arrangements, either X-X 90º/180º
- Cis isomer: same side (90º), Trans isomer: opposite (180º)
 Geometric isomers are not superimposable, different isomers have different functions
Geometric isomers are not superimposable, different isomers have different functions- Coordination # 5 is trigonal bipyramidal or square pyramidal
- Coordination # 6 is octahedral
- If arrangement is [MA2B4]
- Cis or trans for the A ligand
- If arrangement is [MA3B3]
- Facial (fae) isomer: 3 of the same ligands lie on the same face
- Meridian (mer) isomer: 2 are trans & 1 is cis to these
- If arrangement is [MA2B4]
![]() Carbonyl Complexes
Carbonyl Complexes
- Ligands are CO molecules (carbonyl group) (liquid @ room temp., volatile)
Porphyrins
- Type of ligand, the metal atom bonds to the middle (Fe2+), basic structure
has a charge of -2 - N donor atoms coordinate to the metal center, 2 more ligands can bind to
above and below to form octahedral geometry
Hemoglobin
- Transports oxygen from the lungs to the rest of the body
- Tetrahedral shape
- Subunits composed of a protein (globin) linked to a heme (Fe2+ porphyrin) group in a square planar configuration
- The 5th coordination is a histidine side chain (amino acid globin) which produces O2 when dissolved
- The 6th coordination can be O2
- Hemoglobin is made up of 4 polypeptide chains (4 heme groups), each heme group has 1 Fe2+ atom
- Uses Le Châtelier’s principle to release the O2 in deoxygenated areas
- inefficient, large molecule to carry small O2 molecule
- CO is an competitor to O2, the brain becomes oxygen deprived
3.2
Crystal Field Theory: electrostatic field of the ligands
Assumptions:
- Transition metal ion is treated as a free ion (not bounded to ligands)
- The e- are treated as point charges
- Ligands are negatively charged
Orbital Energies (d Orbital Splitting)
- For a free metal ion, all d orbitals are degenerate (same energies)
- If the metal is part of a octahedral complex, the energy levels are not the same as free metal ion because the environment is different
- In octahedral complexes is not spherically symmetrical, therefore the 6 point charges are in an octahedral arrangement along the metal-ligand bonding directions
- dxy, dxz, dyz (point between the ligands) are degenerate, called t2g orbitals (lower energy)
- dz2, dx2-y2 (point directly at ligands) are degenerate, called eg orbitals (higher energy)
- the energy separation between eg and t2g orbitals is Δo (‘o’ refers to octahedral)
- total energy is unchanged from barycenter (energy in spherical field) “if something goes up, something must go down”
Factors Affecting Δo
- Oxidation State of Metal Ion: increases with increasing charge of the metal
 Identity of the Metal: increases going down a group
Identity of the Metal: increases going down a group- Nature of the ligand: increases along
spectrochemical series - Geometry of the complex: the d orbital
splitting pattern of an octahedral
complex is different from that of a
tetrahedral/square planar complex
![]() Colours of Transition Metal Complexes
Colours of Transition Metal Complexes
- Our eyes see the colour complementary to the colour that is absorbed
- RGB Colour Model
- Light in the visible spectrum excites e- from a t2g to eg orbital
v is frequency
Absorption Spectroscopy (UV-visible)
- Colours and colour intensities absorbed can be measured by passing light through it
Magnetism in Transition Metal Complexes
- more unpaired electrons = more paramagnetism
- High-Spin Configuration: fill all orbitals before doubling up
- Low-Spin Configuration: fill all the lower energy orbitals before the higher energy levels
- If Δo is small it doesn’t require that much energy to get into a higher energy level, therefore e- would rather jump up than pair up (pairing requires more energy
- Pairing energy: required to overcome the repulsion between 2 e- occupying the same orbital
- More stable when least repulsion