Lesson 10: Chemical Analysis
Qualitative Analysis
Qualitative analysis is identification to the identity of substance present within a sample. We will look at three areas of study for this.
- Sequential Chemical Analysis
- Flame Colour
- Colour of Solution
Sequential Chemical Analysis
Sequential chemical analysis utilizes precipitation reactions to identify the presence of ions. In doing so, new ions are introduced to create a precipitate and thus identify the presence of existing ones.
This process needs to be well thought out, with some indications of possible ions present. It is set up as a dichotomous key/flow chart, with yes or no questions. As ions cannot be introduced by themselves they need to be coupled with an ion that will not create a precipitate. When adding a cation couple it with the nitrate polyatomic ion (NO3). When adding an anion, couple it with sodium. Both of these ions have high solubility in all cases.
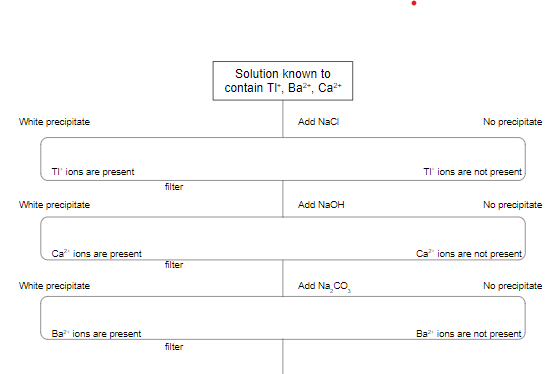
Sequential Chemical Analysis Example
Design an experiment to analyze a single sample of a solution for any or all of the Tl+(aq), Ba^2+(aq), and Ca^2+ (aq) ions.
Step 1 - from solubility chart/table identify all the substances that have low solubility with the suspected ions. Make individual Lists
Tl+; Cl-, Br-, I-, S^2-, CO3^2-, PO4^3-, SO3^2-,
Ba^2+; SO4^2-, CO3^2-, PO4^3-, SO3^2-,
Ca^2+; OH-, SO4^2-, CO3^2-, PO4^3-, SO3^2-,
From the lists, identify an ion that is specific to only one ion \n
Tl+; Cl-, Br-, I-, S^2-, ==CO3^2-, PO4^3-, SO3^2-,==
Ba^2+; @@SO4^2-@@, ==CO3^2-, PO4^3-, SO3^2-,==
Ca^2+; %%OH-%%, @@SO4^2-@@, ==CO3^2-, PO4^3-, SO3^2-,==
In this particular example two ions can be chosen first as they have ions that they would create a precipitate individually, Tl+(Cl-, Br-, I-) Ca2+ (OH-)
Now we can start forming the flow cart
Qualitative Analysis Involving Flame Colour
A limitation of sequential chemical analysis using precipitation reactions is that some cations (alkali metals), are highly soluble (do not form precipitates)
A flame test is a diagnostic test used to identify a specific element. Where a nichrome wire is dipped into an ionic solution and then placed into a Bunsen burner flame. The flame coloured observed is caused by the cation in the solution. Cations are usually coupled with nitrates for this procedure. To the right is a list of expected flame colours by cations.
Using Solution Colour to Identify Ions
| Ion | Solution Colour |
|---|---|
| Groups 1, 2, 17 | colourless |
| Cr^2+(aq) | blue |
| Cr^3+(aq) | green |
| Co^2+(aq) | pink |
| Cu+(aq) | green |
| Cu^2+(aq) | blue |
| Fe^2+(aq) | pale green |
| Fe^3+(aq) | yellow-brown |
| Mn^2+(aq) | pale pink |
| Ni^2+(aq) | green |
| CrO4^2-(aq) | yellow |
| Cr2O7^2-(aq) | orange |
| MnO4^-(aq) | purple |
Most aqueous solutions are colourless (Ions from group 1, 2, & 17 are all colourless. However, many solutions containing monatomic and polyatomic ions of the transition elements do have colour in solution.
The table to the right lists the colours that the ions would have.
Summary
\n \n