Nuclear Physics
Workbook Questions
Questions 5.1
Q1. Protons, Neutrons and Nucleons
a. p = 20, n = 25, nucleons = 45
b. p = 79, n = 100, nuc
leons = 179
c. p = 92, n = 143 , nucleons = 235
d. p = 90, n = 140, nucleons = 230
Q2. Identify each Element
a. Zinc
b. Carbon
c. Silicon
d. Gallium
Q3. How many protons and neutrons
a. p = 27, n = 33
b. p = 94, n = 145
c. p = 6, n = 8
Q4. Difference between stable and unstable isotopes
Isotopes are atoms that have the same number of protons but different numbers of neutrons. Isotopes have the same chemical properties but different physical properties.
Stable isotopes are atoms that have stable nuclei and do not show signs of radioactivity. Their half-life is very long of they don’t have a half-life at all.
Radioisotopes are unstable isotopes that can undergo radioactive decay and are always half-lived. They can be naturally occurring but only for a short time period.
Examples of stable isotopes are Carbon-12, Carbon-13 and Nitrogen-15
Q5. Which atoms are definitely radioactive
U, Pt, Po. Because these three all have a large number of excess neutrons which will most likely cause radioactivity.
Questions 5.2
Q1. Radiation Emissions
a,b,c. Alpha, gamma and beta particles are emitted from the nucleus
Q2. Physical Differences between alpha, beta and gamma particles
Alpha and beta particles have both energy and mass. Gamma rays are pure energy, similar to visible light.
Alpha particles has 2 protons and 2 neutrons while beta particles is a high energy electron. Gamma rays are high-frequency radiation.
Q3. Identify particles
a. Beta Particle
b. Hydrogen atom
c. Helium or alpha particle
d. Boron?
Q4. Atomic and Mass of decayed elements
a. Atom number - 83 & Mass - 205
b. thorium - 88 Protons and 232 Mass
c. e-
d. Mercury e-
Q5. Mode of Radioactive decay
a. Alpha decay
b. Beta decay
c. Beta decay
d. Alpha decay
e. Gamma decay
Q6. Identify the element formed
Lithium 6/3
Q7. Unknown Particles
a.
b.
c.
d.
Q8. Carbon-14 Beta decay Equation
a. electron
b.neutron
c. Carbon-14 → Beta Radiation + Nitrogen-14
Questions 5.3
Heinemann Text Book
5.1 Atoms, Isotopes, and Radioisotopes
Atoms and the Nucleus
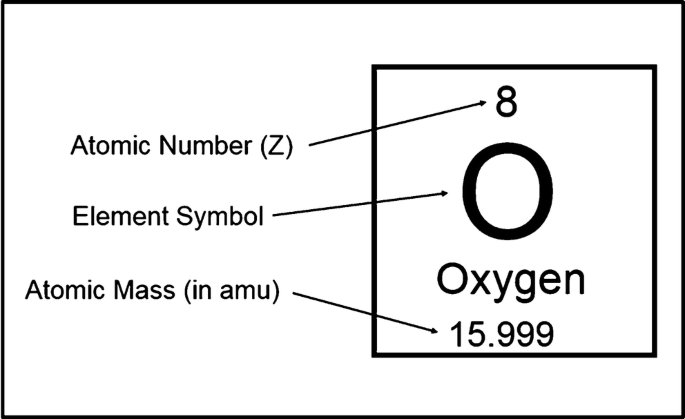
Nucleus - a central part of an atom that consists of protons and neutrons. The nucleus contains nearly all of the mass of the atom, but accounts for very little of its volume.
Nucleons - the collective term for particles within the nucleus; protons and neutrons.
Protons - have a positive charge.
Neutrons - neutral with no charge.
Electrons - Negatively charged particles that are small and light and orbit the nucleus at high speeds.
Atomic Particle | Charge | Mass | Location |
|---|---|---|---|
Protons (p) | +1 | 1 | Nucleus |
Neutrons (n) | 0 | 1 | Nucleus |
Electrons (-e) | -1 | 1/1840 | Orbitals (electron cloud) |
Isotopes and Radioisotopes
Isotopes are atoms that have the same number of protons but different numbers of neutrons. Isotopes have the same chemical properties but different physical properties.
Nuclides - A term when referring to a particular nucleus while ignoring electrons.
An individual radioactive (unstable) isotope is known as a radioisotope. Radioisotopes may spontaneously decay by emitting a particle from the nucleus.
Protons in the nucleus repeal each other due to electrostatic forces. The strong Nuclear Force is a powerful force of attraction between nucleons but only has a short range.
Graph Analysis
As protons increase, more neutrons are needed in relation to protons.
The valley of stability is smaller when there are less protons.
In stable particles neutrons increase in proportion to protons
Alpha radiation is more likely to emit as number of protons increase.
To release alpha radiation, there both must be large number or protons and neutrons and must have relatively less neutrons per proton than its relation to its stable particle
5.2 Alpha, Beta and Gamma Radiation
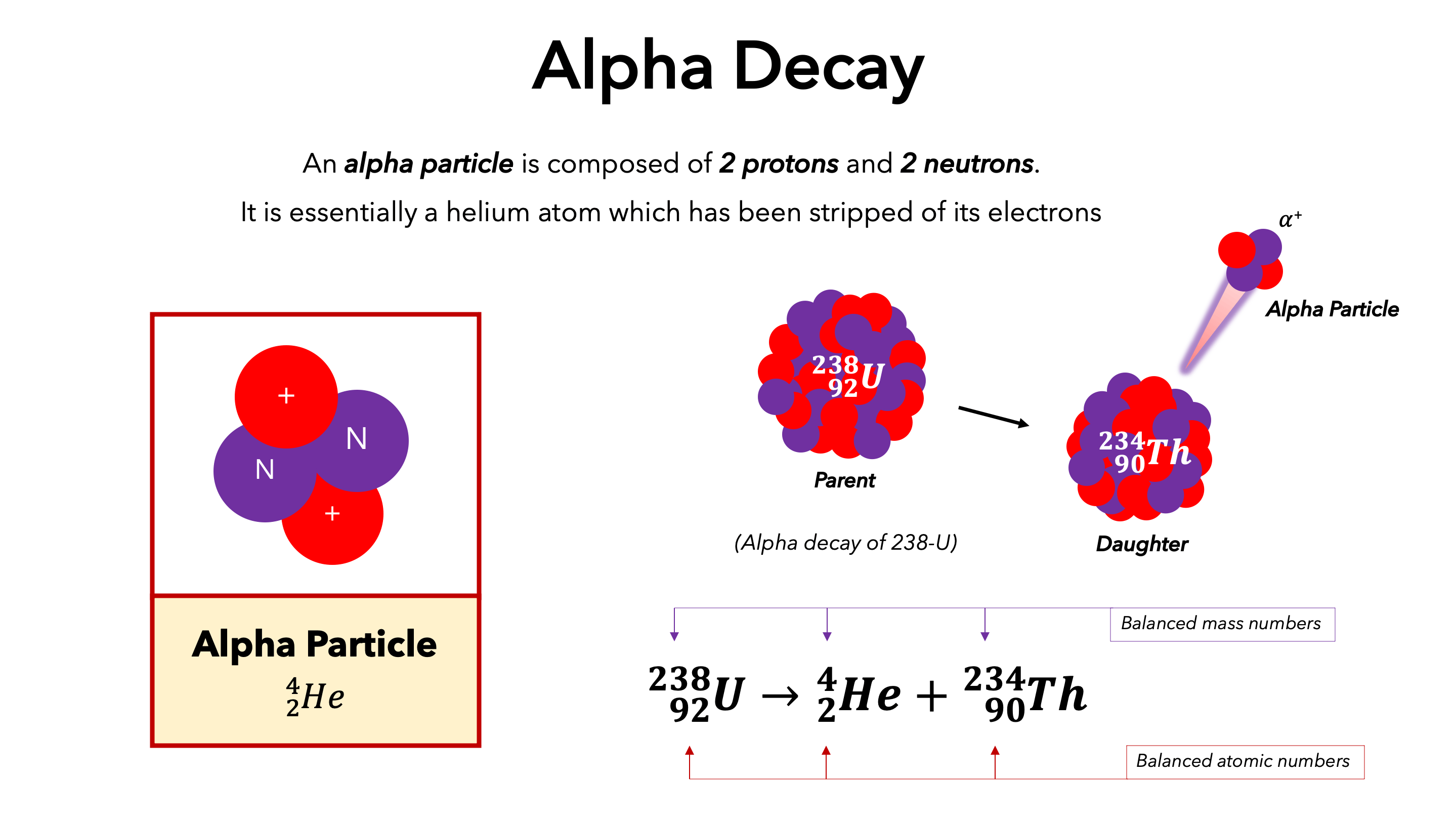
Radioactive isotopes can decay and emit either alpha, beta or gamma radiation from its nuclei. In all nuclear reactions both atomic mass and numbers are conserved.
Decay - occurs when unstable nuclei break down into smaller parts emitting small particles with high energy to become stable.
Parent nuclei - the nuclei that break down.
Daughter nuclei - the resulting nuclei that is left.
A particle has been emitted from the parent nuclei and therefore the nucleus of the daughter nuclei has become smaller. This is a show of alpha/beta decay.
Alpha Particle | Beta Particle | Gamma Particle | |
|---|---|---|---|
Symbol | α or He (2+ or 4/2) | β or e- (0,-1) | γ 00 |
Nature of radiation | Helium nucleus - 2 protons and 2 neutrons | High kinetic energy electrons | High-frequency electromagnetic radiation |
Charge | +2 | -1 | 0 |
Relative mass/size | Heavy/large (must be bigger than Helium) | light/small(only needs to give off an electron) | |
Emitted from.. | Nucleus | Nucleus | Nucleus |
Penetration Depth | 8cm | 1m | Infinite |
Ionising Power | Strong | Moderate | Weak |
Absorbed by.. | Sheet of paper | Sheet of aluminium | Thick sheet of lead or concrete |
Speed | Slow | Fast | Speed of light |
Alpha Decay
Radioactive decay of a heavy nucleus results in an ejection of an alpha particle. Alpha particles are positively charged chunks of matter that consist of two protons and two neutrons.
Beta Decay
An electron that has been emitted from the nucleus of a radioactive atom as a result of a neutron transmuting into a proton. When there are too many neutrons for the nucleus to be stable (in comparison to protons) a neutron will spontaneously change into a proton. An electron and an unchanged massless particle called antineutrino v get ejected in order to restore the nucleus back to a more stable state.
When the neutron transforms into a proton, the product changes (e.g. carbon changes into nitrogen). Atomic mass and numbers stay the same. Antineutrino has no mass or charge so the atomic mass number does not change.
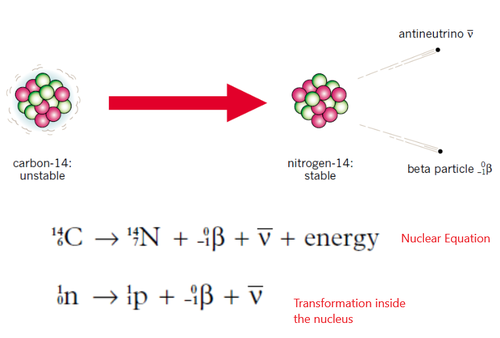
Equations for Beta decay can either be a nuclear equation or an equation that focuses mainly on the transformations taking inside the nucleus.
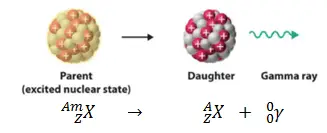
Gamma Decay
In most occasions, after a radioisotope emits an alpha or beta particle, the new or daughter nucleus holds excess energy. The protons and neutrons in the daughter nucleus rearrange slightly to balance the excess energy. As a result of this, gamma radiation, a high-frequency electromagnetic radiation is released. Gamma rays have no mass, are uncharged, and travel at the speed of light.
 Artificial Transmutation
Artificial Transmutation
Around 200 naturally occurring radioactive isotopes (commonly found in earth’s crust or atmosphere). Most of the radioisotopes used in industrial and medical applications are synthesized through artificial transmutation. This process includes bombarding stable nuclei with neutrons.
5.3 Properties of Radiation and Energy Release
Alpha Particles
Because they consist of 2 protons and 2 neutrons they are relatively heavy and slow-moving. They also have a double positive charge (2 protons) and poor penetrating ability. When emitted from the nucleus they reach speeds only up to 10% the speed of light.
When alpha particles travel through the air, they interact and dislodge electrons from nearly every atom it touches. It is the largest in size, double positive charge, has the most mass and moves the slowest. This means that it’ll encounter more atoms and therefore have a high ionising ability.
Beta Particles
Fast-moving electrons are created when a neutron is decayed. When emitted from the nucleus they can reach up to 90% of the speed of light. Beta Particles are more penetrative in comparison to alpha particles as they are smaller with faster charge. A 1mm thick aluminum sheet will stop it.
When beta particles encounter an electron cloud, they are repelled (because they have a negative charge). This means that when Beta particles travel they lose less energy per collision in comparison to a beta particle and are more penetrating but do not ionise as easily.
Gamma Rays
Electromagnetic radiation with very high frequency. It has no mass and travels at the speed of light. No electric charge with very high energy making it very penetrative. They can travel an unlimited distance through air and even a few centimetres of lead or concrete would not completely absorb gamma rays.
No charge and moves at the speed of light. Gamma rays pass through matter very easily meaning that they have a very poor ionising ability but are very penetrative.
5.4 Half-life and Radioisotopes
It’s important to note that different radioisotopes will decay and emit radiation at varying rates. The rate of decay is determined by the instability of the nucleus and is independent to external conditions such as temperature, magnetic fields or the chemical environment.
The half-life of a radioisotope is the time that it takes for half of the nuclei of the sample radioisotope to spontaneously decay. E.g. the half-life of polonium-218 is 3 minutes.
Short Vs Long Half-lives
Radioisotopes with short half-lives decay quickly and are suitable for diagnostic purposes. While those with longer half-lives take more time to decay, making them suitable for therapeutic purposes or determining the age of ancient objects (carbon dating).
Naturally radioactive isotopes usually have a longer half-life.
5.5 Nuclear Fission
Nuclear fission occurs when a nucleus splits into tow or more pieces. This is often triggered by the absorption of a neutron. A relatively large amount of energy is released during the fission process.
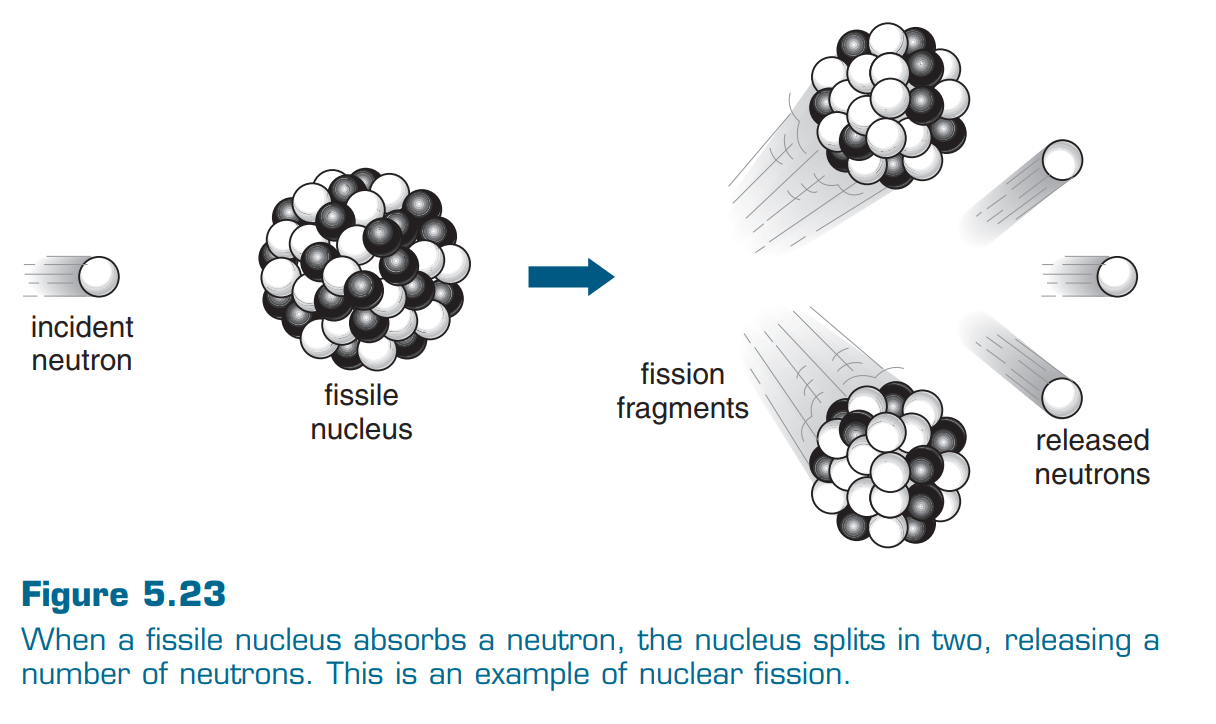
Fissile materials are elements that are capable of undergoing nuclear fission. This includes uranium-235 and plutonium-239 (the most common fuels used for nuclear energy).
5.7 Nuclear Reactors
Objectives
I can recall a description of Bohr’s model of the atom
I can recall what is meant by;
Atomic Number
Atomic Mass
Protons
Neutrons
I can define;
Nucleons
Strong Nuclear Force
Electromagnetic Force
Radioactive decay
I can relate knowledge of strong nuclear force and (counters) electromagnetic force to the stability of the nucleus.
I can relate binding energy to stability of a nucleus and relate to nuclei becoming radioactive.
Practice Questions
Summary Notes - Textbook
Written answers to questions
The mass defect is the mass difference between the rest mass of a nucleus and the sum of the rest masses of its individual constituent nucleus.
2 advantages and disadvantages of nuclear energy:
Produces no polluting gases/greenhouse gases only water vapor. Low fuel costs power station has very long lifetime
disadvantages, waste is radioactive, and thermal pollution form wastewater. large, scale safety concerns high costs to construct and decomposition
Strong Nuclear force - the strong nuclear force holds together the subatomic particles of the nucleus protons and neutrons or nucleons.
Radioactive half-life - the half-life is the time required for the one-half of the atomic nuclei of a radioactive sample to decay.
What makes greatest ionizing ability but least penetrative power
International Atomic Energy Agency (IAEA)
Reliability Assessment: The IAEA is a reputable international organization that promotes the peaceful use of nuclear energy and aims to prevent its use for any military purpose. Articles from the IAEA are generally considered reliable and trustworthy due to the agency's stringent standards and global reputation.
Earth.org
Reliability Assessment: Earth.org is a non-profit environmental organization that provides insights on various environmental topics. While it offers valuable perspectives, it's essential to note that the content may sometimes reflect environmental advocacy perspectives. Always a good idea to cross-check data with other reputable sources.
World Nuclear Association
Reliability Assessment: This is an international organization that represents the global nuclear industry. It provides detailed and technical insights into various aspects of nuclear energy. As it is industry-affiliated, the information might sometimes reflect the industry's perspective. However, its detailed reports and insights are generally considered reliable for factual content.
Nuclear Energy Institute (NEI)
Reliability Assessment: NEI is a US-based organization representing the nuclear technology industry. Like the World Nuclear Association, its insights are valuable, especially for technical details. Still, as with any industry-affiliated organization, it's wise to cross-check data when used in arguments that may have policy or economic implications.
International Energy Agency (IEA)
Reliability Assessment: The IEA is an autonomous organization that provides data, analysis, and solutions on all fuels and technologies. Its reports are well-regarded and often cited in academic research and policy-making due to their comprehensive nature and rigorous methodology.
Office of Nuclear Energy, U.S. Department of Energy
Reliability Assessment: This is a government body that provides insights into the various aspects of nuclear energy from a US perspective. Government sources are typically reliable for factual data, although they may sometimes reflect national policy perspectives.
World Nuclear Association (Repeated)
Reliability Assessment: As mentioned earlier, this is an industry-affiliated source. Still, its detailed reports and insights are generally considered reliable for factual content.
Conserve Energy Future
Reliability Assessment: This appears to be an environmental advocacy website. While it can provide valuable insights, especially from an environmental perspective, it's always a good practice to cross-check data and ensure a balanced viewpoint.
World Nuclear Association (Repeated)
Reliability Assessment: As previously stated, this industry-affiliated source offers valuable technical insights but should be cross-referenced with other sources when necessary.