CHEM 154 Final Review (Learning Objectives)
Unit 1: Highschool Review
Difference between valence and core electrons:
Valence: Electrons in the outermost shell.
Core electrons: Do not participate in chemical bonding. Not in the outer shell.
Determining the number of valence electrons and core electrons based on electron configuration for atoms and ions:
Electrons in the outer most sub-shell are valence electrons.
What is Zeff? (Effective Nuclear Charge) Pattern?
Average nuclear charge felt by an individual electron in an atom, taking into consideration shielding
Zeff = Z - S (Z = # protons in nucleus) (S = Inner shell electrons)
What is atomic and ionic radius? Pattern?
Atomic radius: Size increases going left and down the table.
This is because as you go down a group, a new shell is added, and at the number of electrons in the valence shell decreases.
Ionic Radius:
Cations have a smaller radius. Larger positive charge, the smaller. Due to electron attraction.
Anions have a larger radius. Larger negative charge, the bigger. Due to electron repulsion.
What is ionization energy? Pattern?
Ionization energy is the amount of energy required to pull an electron from the atom/ion.
Fuller outer sub-shell, greater ionization energy
Increases going down shells
Eion = (kQ1*Q2)/r
What is electron affinity? Pattern?
Adding an electron to a gaseous atom releases a lot of energy, very exothermic.
Energy change that occurs when electron is absorbed by a gaseous atom.
Higher electro negativity = higher electron affinity
Generally becomes more exothermic going right
What is electronegativity? Pattern?
Ability of an atom to attract an electron to itself.
Increases going up and right the PT.
M like to give away electrons, NM like to attract electrons.
Ionic vs Covalent bonds
Ionic
Donate electrons
Stronger that covalent bonds
Covalent
Share electrons
Weaker than ionic bonds
How can you predict the nature of a chemical bond? (ionic/covalent, polar/non polar)
Compare electro negativities
Pure covalent (Non-Polar): ΔEN < 0.4
Polar covalent 0.4 < ΔEN < 1.8
Ionic ΔEN > 1.8
What is lattice energy? What is the trend in solids?
Amount of energy needed to separate a mole of solid ionic compound into its gaseous ions
Ex: NaCl(s) → Na+(g) + Cl-(g)
↑ Charge ↓ Ion Size = ↑ Lattice Energy (More attraction)
↓ Charge ↑ Ion Size = ↓ Lattice Energy (Less attraction)
How to find which compound has the higher Lattice Energy?
Multiply the charges of all the ions in the compound and take the absolute value.
Unit 2: Lewis Structures
How to draw Lewis Structures?
Count number of Valence E
Least electro negative is generally in the centre
Use all valence electrons
Label Formal Charges
Most stable Lewis Structures:
Least non-zero FC’s
Negative charge on the most electronegative atom and positive on the least electronegative atom.
Lewis Structures: Octet Rule
Elements must have 8 surrounding valence electrons
Lewis Structures: Duet Rule
Hydrogen must be surrounded by 2 electrons.
Radical Species:
Molecules with an odd number of electrons (unpaired electron)
Hyper Valence (Expanded Octets):
Elements in the third row can expand their octet
(Breaks octet rule)
What is formal charge?
Difference between number of valence electrons and number of electrons surrounding an at0m in a particular Lewis Structure
Formal Charge = Valence E - Lone Pair E - Bonding
What is the overall molecular charge?
Sum of FC = Overall Molecular Charge
What are Resonance Structures?
Same arrangement of atoms, different arrangement of electrons.
How to make/draw resonance structures?
Lone pairs and double-bond electrons move around
Unit 3: VSEPR Theory
VSEPR Theory
Predicts molecular shape as point-charges want to be as fr from each other as possible.
Determining Molecular Polarity:
Are there any lone pairs on the central atom?
Yes: Polar
No: Are the lone pairs on the surrounding atoms equal?
No: Non-polar
Yes: Polar
Unit 4: Intermolecular Interactions & Phases of Matter
Intermolecular Forces:
Intermolecular forces are the attractions between molecules.
Types of Intermolecular forces
London Dispersion
Exist in all atoms
Atoms temporarily inducing dipoles
Weakest force
Dipole-Dipole
Molecules with a permanent dipole
Strong force
Hydrogen Bonding
Molecules with H bonded to N, O, F.
Strong Force
Charge-Charge (ion-ion)
Very strong force
Ionic solids or ionic liquids
Charge-Dipole (ion-dipole)
What is Polarizability?
How easily an electron cloud can be disturbed by an electron field
Larger atoms/molecules are more Polarizable
Relationships between intermolecular forces and:
Melting point
Stronger intermolecular forces, more energy, higher melting point
Boiling point
More branching of central atom, lower boiling point
Vapor Pressure
Stronger intermolecular forces, lower rate of evaporation, lower vapour pressure
How to predict the types of intermolecular forces in a particular substance?
Lewis Diagrams
H - FON
Hydrogen Bonding
Dipole Dipole
London Dispersion
Polar Molecules
Dipole Dipole
London Dispersion
Non-Polar
London Dispersion
Interpreting Phase diagrams:
Critical point
Liquid and gas phase are indistinguishable
Phase Changes:
Trends from going Gas → Liquid → Solid
Average IMF increases
Molecular Spacing decreases
Entropy decreases
Sublimation
Solid to vapour
Deposition
Vapour to gas
Melting
Solid to liquid
Freezing
Liquid to solid
Vaporization
Liquid to vapour
Condensation
Vapour to liquid
Thermodynamic Equilibrium
No net macroscopic flows of matter of energy within a system or between system and surroundings
Macroscopic properties of system remain constant
Unit 5: Polymers
What is a monomer?
Small molecules
What is a polymer?
Molecule built up from monomers
Short-Hand notation
Find where polymer pattern is repeating
Take that part and put brackets around it
Bottom right, outside the bracket write the letter ‘n’.
n = number of monomers
What is a oligomer?
An oligomer is a molecule that consists of a few monomer units
What is a degree of polymerization?
Amount of repeating units in a polymer chain
Structure | Degree |
|---|---|
Dimer | 2 |
Trimer | 3 |
Tetramer | 4 |
Pentamer | 5 |
Oligomer | Small |
Polymer | Large |
What is crosslinking?
Formation of covalent bonds that hold together polymers
What is elastomers?
Can be stretched with little or without permanent deformation
How to draw and interpret Line Bond Structures?
Covalent bonds are the lines
Unless specified, end of the lines represents a carbon atom
Each carbon is bonded to H atoms, unless otherwise stated
Lone pairs can be omitted
How to draw and interpret Condensed Lewis Structures?
Condensed lewis structures leave out lone pairs and outer bonds.
Functional Groups
Group of atoms with distinct properties
Types of Functional Groups:
Name of Functional Group | Structure | |
|---|---|---|
Carboxylic Acid |
| |
Amine |
| |
Amide |
| |
Acid Chloride |
| |
Alcohol |
| |
Ester |
|
Types of Polymer Linkage
Addition
Two or more molecules join and create a larger molecule without the loss of atoms/molecules
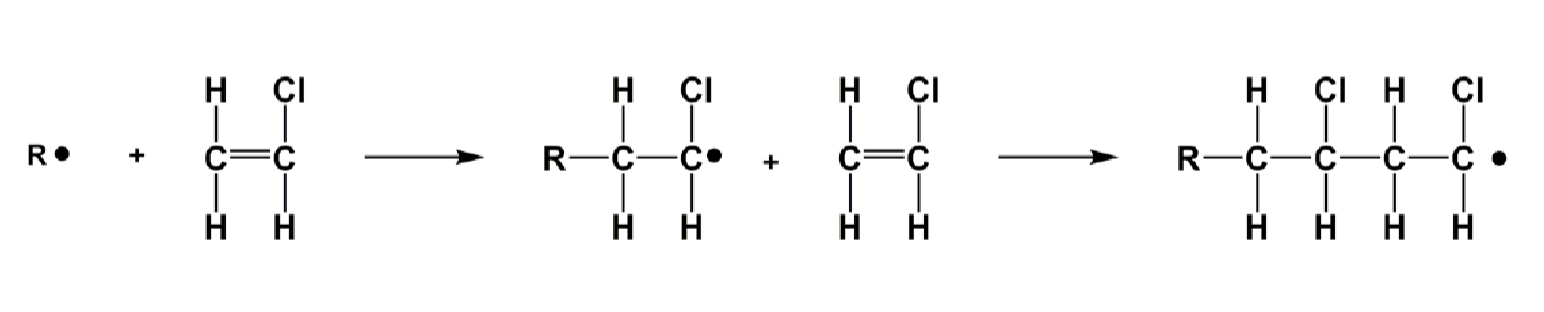
Polymerization:
Initiation
Number of radicals increases
Starts with the formation of a radical (Refer to unit 1) that is very reactive and an odd number of electrons
Abbreviated as R.
Propagation
Number of radicals remains constant
Growing polymer reacts with monomeric unit, growing the polymer.
Termination
Number of radicals decreases
There is a reaction between a growing chain and another radical species
Condensation:
Amide linkage
Carboxylic acids or an acid chloride react with AMINES
Two monomers join and form a molecule byproduct (water or HCL)
Ex/ OH +H2N forms a byproduct of water
Ex/ Cl + H2N forms byproduct of HCL
Ester linkage
Carboxylic acid or an acid chloride reacts with ALCOHOLS
Forms byproduct of water or HCL
Reactant 1
Reactant 2
Linkage
Byproduct
Carboxylic Acid
Amine
Amide
Water
Carboxylic Acid
Amine
Amide
HCL
Acid Chloride
Alcohol
Ester
Water
Acid Chloride
Alcohol
Ester
HCL
What affects polymer properties?
Polymer architecture
Linear
Stronger
Can pack together easily
Branched
Weaker
Cannot pack together easily
Molecular weight
Greater molecular weight, mechanical strength increases
The lower the molecular weight, lower the transition temperature, viscosity, and the mechanical properties
Crosslinking
High-stiffness
Stronger
**What is weight distribution?**
Unit 6: Gases
Ideal Gas Law:
→ PV = nRT
Units
Pa, m³, 8.31 j/mol*K
kPa, L, 8.31 j/mol*K
arm, L, 0.08206 L*atm/K*mol
Ideal vs Real gases
Ideal:
High temperatures and Low Pressures
Molecules do not interact
Accurate at low pressure because of low interactions
Not accurate at high pressures, more interactions
Real:
Molecules do have interactions
Accurate at high pressures.
How to use the Van der Waals equation
(P + an²/V²)(V - nb) = nRT
a = constant for strength of attractions
b = constant for size of gas particles
Dalton’s Law of partial pressures
Ptotal = P1 + P2 + P3 + P4…
Mole Fraction
Ratio of number of moles in a mixture to total number of moles.
Kinetic Molecular Theory:
Gas is made up many particles in constant random motion
Gas particles occupy no volume
Collisions are elastic
Only interact during collisions
Average KE is proportional to temperature
When temperature increases
Average KE increases
Unit 7: Energy and Chemistry
First law of thermodynamics
Explanation
Because heat and work account for all energy exchange between a system and surroundings, energy change must equal the change in the total energy of a system
Equation
ΔU = Q + W
Hess’s Law
Sum of ΔH for all steps gives overall reaction enthalpy
ΔH = State Function
What is ΔH
Enthalpy
Equal to ΔU
Work
Object does work on a system = NEGATIVE EXOTHERMIC
System does work on the object = POSITIVE ENDOTHERMIC
What is it?
Energy transferred between system and surroundings
PV work
W = -PextΔV
If ΔV is positive
Work is done by system → Negative
if ΔV is negative
Work is done on system → Positive
Extensive vs Intensive Properties
Extensive
Scaled with the size (QUANTITY) of the system
Intensive
Does not scale with the size (QUANTITY) of the system
Heat capacity
Quantity of heat required to change the temperature of a substance
Molar heat capacity → Cpm (mols)
Specific heat capacity → Cp (grams)
ΔU: Internal Energy
Total energy of particles in a system
Unit 8: Entropy and the Second and Third Laws of Thermodynamics
Second Law of Thermodynamics
Reactions increase the entropy in the universe
Third Law of Thermodynamics
The entropy of a system approaches a constant value as the temperature approaches absolute zero
What is Entropy?
The amount of disorder in a system
If increasing, reaction is irreversible
What is Enthalpy
Amount of internal energy in a compound
Deduce the sign of ΔS for many chemical reactions by examining the physical states of the reactants and products.
How to determine whether Entropy is increasing or decreasing?
Increasing
Number of gas moles increasing
Temperature increases
\Increasing volume
Going from a more ordered state to disordered (solid to liquid to gas)
Decreasing
Number of gas moles decreases
Going from a disordered state to more ordered (gas to liquid to solid)
S = klnW
What is this formula?
k is the Boltzmann constant
W = is the number of arrangements that are possible in the system
ΔSuniverse = ΔSsurr + ΔS >= 0
ΔS can be positive or negative, but ΔSuniverse must NEVER be negative.
ΔSuniverse = 0 if all processes are reversible
Standard Molar Entropy
State function.
ΔS(knot) = vSproducts - vSreactants (v = coefficient)
What is ΔG?
Predicts spontaneity of reaction
ΔH | So | -TΔSo | ΔG | Spontaneous? |
|---|---|---|---|---|
+ | - | + | + | No |
- | + | - | - | Yes |
- | - | + | + or - | At Low Temp |
+ | + | - | + or - | At High Temp |
Ecell positive = spontaneous
Negative = non-spontaneous
ΔG0 = ΔH0 - TΔS0
Used to calculate Gibbs
Unit 9: Chemical Equilibrium
Describe the relationship between ΔG°, ΔGrxn, Q, and K,
and and apply this relationship to gain information about
chemical reactions.
What is K?
K is the way to determine which way the reaction shifts
Large K → products are favoured, goes to completion
Small K → reactants are favoured, reactants are favored
K close to 1 → around equilibrium
ΔGrxn = ΔG0 - RTlnQ
Difference between this and ΔG0 = ΔH0 - TΔS0
ΔG0 = ΔH0 - TΔS0 is for reactions under non-standard conditions (any conditions)
ΔGrxn vs ΔG0
ΔGrxn | reaction is: |
|---|---|
less than 0 | spontaneous in fwd direction |
0 | at equilibrium |
greater than 0 | spontaneous in rev. direction |
ΔG0 tells you about equilibrium position
ΔGrxn tells you which way it’ll proceed to reach equilibrium
At equilibrium equals 0
Therefore:
ΔG0 = - RTlnK
K = Q at equiibrium
ln(K2/K1) = - ΔH0/R (1/T2 - 1/T1)
Assuming ΔH knot doesn’t change with temperature we can use this formula.
Solubility Ice Tables and Keq
Keq = [products]/[reactants] to the power of their coefficients
Solids and liquids are not in the eq expression
– Differentiate between a reaction quotient and equilibrium
constant and describe the meaning of both.
Reaction Quotient Q vs Keq
The reaction quotient (Q) is a way to measure the relative amounts of products and reactants present during a reaction at any given moment. It's like a snapshot of the reaction's progress.
Q compares the current situation to the perfect recipe. If Q doesn't match K, the reaction isn't at equilibrium yet, and something will change to try to get there.
Unit 10: Chemical Kinetics
Define the rate of a chemical reaction and express the rate in terms of the concentrations of individual reactants or products.
Use the method of initial rates to determine rate laws from experimental data.
Use graphical methods to determine rate laws from experimental data.
Use the rate-determining-step to calculate reaction rates in multi-step reactions.
Relate the qualitative principles of collision theory to the quantitative treatment of rates by the Arrhenius equation.
Calculate the activation energy for a reaction from experimental data.
Explain the role of a catalyst in the design of practical chemical reactions.
Explain the importance of both kinetic and equilibrium considerations in the design of industrial processes.
Factors that affect Reaction Rate
Heterogenous reactants
Reactants that have different states (one gas one solid)
Homogenous reactants
Reactants that have the same state (both gases)
Surface Area
Concentration
Pressure (gasses)
Nature of Elements
Catalysts
Increase in Temp
Role of Catalysts:
Speed up the reaction
Decrease the activation energy required
Reaction Rate: k[A]x[B]y
A and B are molarity of reactants
x and y are their order of reaction
Elementary and Intermediates
Intermediate
Is formed and consumed in the reaction
Unit 11: Electrochemistry
Oxidation Number Rules
Neutral compound
0
Ion
Oxidation number adds up to charge on the ion
Free Elements
0
Fluorine
-1
Group 1 = +1 Group 2 = +2
Hydrogen with Non-Metals
+1
Hydrogen with Metals and Boron
-1
Oxygen except with Fluorine or Peroxides
-2
Group 17 = -1
Group 16 = -2
Group 15 = -3
LEO GER
Loss E Oxid
Gain E Reduc
RED CAT POS
Reduction is at Cathode which is positive
Writing ElectroChemical Equations
Electrolytic |
|---|
2Na+ + e- → 2Na (reduc) |
2Cl- → Cl2 + 2e (oxid) |
2Na+ + 2Cl → 2Na + Cl2 |
Ecell = E cathode - E anode
Ecell=Ecell∘−nFRTlnQ
ΔG0 = -nFE0cell
F = Faradays constant
96485 C mol-1