Carbs
carbohydrates:
- these include sugars, starch, glycogen, and cellulose and they only contain carbon, oxygen, and hydrogen
| Type of carbohydrates | features |
|---|---|
| Monosaccharides | Simple sugars that only contain one molecule. E.g triodes, pentodes, hexoses |
| disaccharides | Two simple sugars chemically linked by glycosidic bonds formed due to a condensation reaction Sucrose = glucose + fructose Lactose = glucose + galactose maltose = glucose + glucose |
| Polysaccharides | Many simple sugars linked by glycosidic bonds Starch = energy store in plants Glycogen = fuel store in animals Cellulose = in plant cell walls |
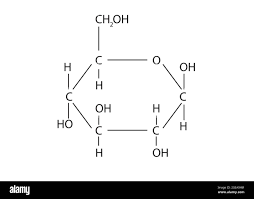
structure of glucose:
- has the molecular formula of C6H12O6
- the carbon atoms are numbered from 1 on the far right, to 6 at the top
The isotopes of glucose:
Alpha glucose, where both the OH groups are ‘below the ring’
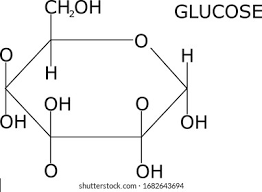
Beta glucose, where one OH is below and the other is above ‘the ring’
Testing for glucose:
- using Benedict’s solution which is actually copper (II) sulphate which has a blue colour
- When a reducing sugar is heated with Benedict’s, the COOH group in the molecule reduces the Cu2+ ion to Cu+ ions which formed a brick red precipitate of copper (I) oxide.
- There is a range of results from blue → green → yellow → brown → red
disaccharides:
Are made of two monosaccharides jointed together by glycosidic bonds that form from condensation reactions and produce water (hydrolysis uses water to break these bonds in the opposite reaction)
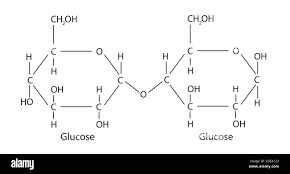
- the glycosidic linkage occurs between C-1 and C-4 of the alpha glucose molecule
polysaccharides:
Are polymers made up of many monosaccharides linked together by glycosidic bonds formed during condensation reactions.
Polysaccharides are macromolecules as they’re giant molecules.
starch:
is a mixture of 2 polysaccharides, both of which are polymers of alpha glucose
- Amylose - unbranched chain of alpha glucose
- Amylopectin - highly branched chains of alpha glucose
The molecule is highly coiled due to the 1-4 glycosidic bonds in amylose and highly branched due to the 1-6 and 1-4 glycosidic bonds in amylopectin
Starch is a major store of energy in plants and therefore has to be compact.
It’s useful as it is compact and insoluble so it doesn’t have an osmotic effect
Starch is fast at being hydrolysed in order to produce energy which is used in respiration to form energy due to the highly branched nature of it
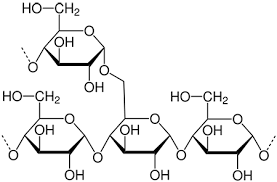
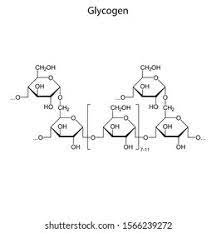
glycogen:
is a polymer of alpha glucose
It is very similar to amylopectin, but is larger and has more branches
Glycogen is found in the liver cells and the muscle cells and is a energy store in humans and animals
Glycogen is very quickly hydrolysed due to the branching that forms because of the 1-6 and 1-4 glycosidic bonds between 2 molecules of alpha glucose
Glycogen is branched after every 10 glucose residues where as amylopectin is branched every 30 glucose residues (residues = when monomers are linked together in a polymer)
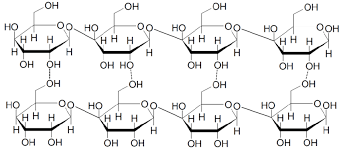
cellulose:
is a polymer of beta glucose
Cellulose is a very straight and unbranched molecule
This is because every other beta glucose molecule is inverted which allows the 1-4 glycosidic bonds to form in a straight way that produces long straight chains
The chains naturally become packed into fibres which are held together by hydrogen bonds - these strong fibres that are arranged in different directions are what make plant cell walls strong and able to withstand the hydrostatic pressure