Radioactive Materials (OCR)
Atom Structure
Electrons, protons, and neutrons
Atoms have a radius of about 1 × 10−10 meters, making them extremely small. Small molecules have a minimum ten-fold bigger size.
A nucleus made up of protons and neutrons with smaller electrons orbiting outside the nucleus is how the atom is currently understood.
The nucleus' radius is significantly lower than the atom's.
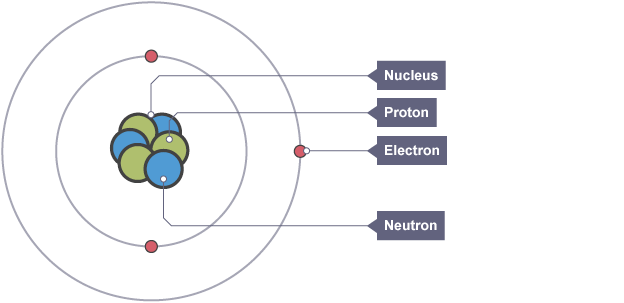
Every particle is unique in terms of both mass and charge:
Relative charge
Proton: +1
Neutron: 0
Electron: -1
Relative Mass
Proton: 1
Neutron: 1
Electron: close to 0 (1/2,000)
Atomic and mass numbers
Since protons and neutrons are the heaviest particles in an atom, they account for the majority of the atom's mass.
Electron mass is frequently regarded as insignificant.
An atom with six protons in its nucleus will always be carbon, while uranium will always have 92 protons. This is because the number of protons in an atom specifies the element.
Atomic models over time
The word atom is derived from the ancient Greek word "atomos," which means "indivisible."
According to the Greek philosopher Demokritos (460–370 BCE), matter could always be split and subdivided into smaller and smaller components until there was a particle that could never be split any smaller. He called this an atom.
Dalton's theory of the atom
The concept of the atom was not significantly altered until English chemist John Dalton (1766–1844) came to the following conclusion:
Atoms, the building blocks of all matter, are unbreakable and incapable of disintegrating.
Every atom in a given element is unique from every other atom and identical to every other atom in the element.
His conclusions were based on gas combination experiments. He discovered that the atoms from the first and second elements only combine in small, whole number ratios, such as 1:1, 1:2, or 2:3, if two elements can be combined to generate a variety of compounds.
Plum pudding model
When J. J. Thomson discovered the electron in 1897, he hypothesized that the atom resembles “plum pudding”.
He proposed that the atom was made of positive "dough" with a large number of negative electrons trapped in it in order to explain the two forms of static electricity.
This was in line with the information at hand at the time:
Since solids cannot be crushed, all of its constituent atoms must be solid.
Rubbing two solids together frequently produces static charge, indicating that there must be an external material (electron) on atoms that may be transferred when they meet.
Nucleus and Rutherford
Ernest Rutherford tested the plum pudding model in an experiment in 1905. A very thin gold leaf held in a vacuum was the target of a beam of alpha particles guided by his two students, Hans Geiger and Ernest Marsden.
After doing their experiment, the scientists noted the following:
The majority of the alpha particles flowed through the foil unhindered.
A tiny proportion of alpha particles that were traveling through the foil were deflected at considerable angles (>4°).
A very tiny quantity of alpha particles bounced back off the foil.
In light of these findings, Rutherford came to the following conclusion:
The majority of alpha particles passed through the foil without difficulty, providing evidence that the atom is primarily made of empty space.
Deflecting a tiny number of alpha particles at large angles indicated that the atom contained a concentration of positive charge. Positive charges were repelling the positive alpha particles because like charges repel like charges.
The extremely small number of alpha particles that returned exactly indicated that the atom's nucleus, or tiny volume, contains all of the positive charge and mass. This small number indicated that there was very little probability of being on that precise crash trajectory; hence, the 'target' needed to be very small as well.
Rutherford had found the “nuclear atom”, a tiny, positively charged particle. A layer of negatively charged electrons forms the exterior of the atom, encircling the nucleus, which is surrounded by empty space.
Bohr and the energy levels
Despite the fact that Rutherford had established the nucleus' existence, scientists remained uncertain about how electrons fit into this new framework.
Niels Bohr updated Rutherford's model in 1913, proposing that the electrons orbited the nucleus at distinct energies or separations from it.
As a result, he was able to clarify that since specific chemicals burn with flames of a specific color, the energy produced by electrons during a chemical reaction must be consistent for each and every atom of that element.
As a result, within each sort of atom, electrons must have defined levels of energy rather than being organized randomly.
Most people today conceptualize atoms in terms of Bohr's "solar system" model.
Isotopes
Using atomic symbols
The two most crucial pieces to know about an atom are its mass number and atomic number.
One way to represent an atom is via symbol notation:
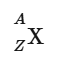
Where:
The mass number is A.
The atomic number is Z.
X is the element's symbol.
Chlorine (Cl), for instance, can be represented as:

This symbol indicates that the nucleus of chlorine has 35 particles, 17 of which are protons and neutrons.
It also indicates that chlorine has 18 neutrons (35 - 17) and 17 electrons because an atom of a neutral state has the same number of protons and electrons.
Isotopes and atoms
An element is defined by its atomic number. Chlorine is and always will be an element with 17 protons.
On the other hand, an element's mass number can change, meaning that its neutron content can also change.
Chlorine can therefore have a mass number of 37, which indicates that it has 20 neutrons, or a mass number of 35, which indicates that it has 18 neutrons. Isotopes are the different forms of chlorine.
Radioactive decay
Steady nuclei
A particular ratio of neutrons to protons is necessary for the stability of an atom's nucleus.
If an element has the same amount of neutrons as protons, even if it has fewer protons—like the elements toward the top of the periodic table—it is stable. For instance, stable carbon (carbon-12) has six protons and six neutrons.
To keep the nucleus stable, more neutrons are required as the number of protons rises. Lead, for instance, has 124 neutrons and 82 protons (lead-206).
While naturally occurring, excessively or insufficiently neutron-rich nuclei are unstable and will decay by radiating out energy.
Radioactive decay types
An unstable nucleus may decay by releasing a single neutron, an alpha particle, a beta particle, or a gamma ray.
Alpha particles
An alpha particle, which is a 'package' consisting of two protons and two neutrons, is released by the nucleus when its neutron count is low.
The nucleus experiences a fourfold fall in mass number and a twofold decrease in atomic number due to alpha decay.
Beta particle
A neutron will become a proton and release a fast-moving electron if the nucleus contains an excessive number of neutrons.
This process is known as beta radiation, and the electron is called a beta (β) particle.
Although the beta particle is an electron, it originates from the nucleus rather than the exterior of the atom.
While it is not common knowledge that electrons are found in nuclei, neutrons can split into two different forms:
a positive proton, which has the same mass as an electron but a positive charge, and a;
electron, which has a negative charge to balance the positive charge. The electron is then ejected quickly and with a large amount of energy.
Gamma ray
The nucleus will frequently still be too "hot" after releasing an alpha or beta particle, and it will lose energy similarly to how a hot gas cools down. An electromagnetic wave called infrared radiation is released by a heated gas to cool it down.
When high energy particles decay to lower energy levels, they release energy. The nucleus will release gamma rays, which are more intense electromagnetic waves, in order to cool down because their energy levels are significantly higher than those of the gas.
The atomic number and mass number of a nucleus stay constant because gamma ray emission has no effect on the number of particles within the nucleus.
Emission of neutrons
Radioactive decay can occasionally release a neutron into the atmosphere. Neutron emission from the natural absorption of cosmic rays high in the atmosphere is a rare occurrence near the Earth's surface, although it can happen.
Alternatively, it can happen artificially. For example, when alpha particles are fired at beryllium, neutrons are released.
Nuclear fission reactions provide an additional instance of neutron emission, as the splitting of the parent nucleus releases neutrons.
Nuclear formulas
Alpha or beta particles are released by a nucleus when it transforms into a new element. Nuclear equations are used to characterize these changes.
The element's mass number is reduced by four and its atomic number is reduced by two as a result of alpha decay, which produces two protons and two neutrons. A helium-4 nucleus is the same as an alpha particle.
Example:
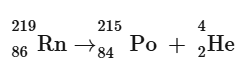
The nucleus receives a proton as a result of beta decay, changing the atomic number by one, but the mass number stays the same since an electron is ejected along with the proton, meaning that a beta particle is an electron.
Half-life
The process of radioactive decay is random. The number of nuclei in a block of radioactive material is in the billions. One cannot predict the exact time at which a certain nucleus will decay because not all nuclei are likely to decay simultaneously.
Since there are so many nuclei, it is conceivable to predict that a specific number will decay within a specific amount of time, even though it is impossible to predict which specific nucleus will decay next.
Scientists can use statistical techniques to determine when 50% of the unstable nuclei in a sample will have been destroyed, but they are unable to predict when a specific nucleus will disintegrate. This is called the half-life .
The number of decays that a detector, like the Geiger-Müller tube, records every second is known as the count-rate.
Radiation types
It is common practice to compare the various forms of radiation according to their airborne penetration, ionization, and travel distances.
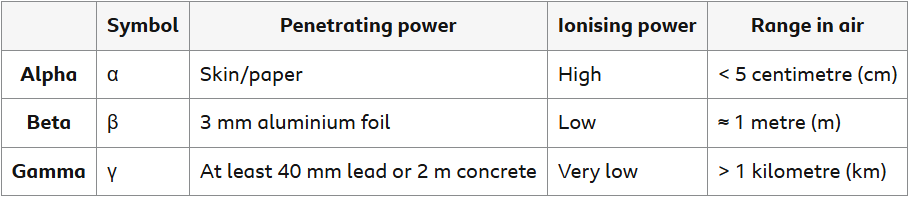
Alpha radiation is used, for instance, in smoke detectors. Because alpha radiation has a very strong ionizing power and a very short range, an alpha radiation source is used.
Contamination and Irradiation
Irradiation
A hand exposed to visible radiation from a torch beam will glow since it has been exposed to light.
Irradiation is the process of subjecting items to radiation rays. The phrase refers to all forms of radiation, including atomic nucleus radiation.
Irradiation from radioactive decay can harm live cells. This is both dangerous and useful at the same time.
Contamination
When radioactive materials are added to an object, contamination occurs. An apple that has been injected with cobalt-60 is contaminated, whereas an apple subjected to cobalt-60 radiation is irradiated.
Similar to radiation, contamination has both possible benefits and drawbacks.
Contamination versus Irradiation
It is common to mix the two processes of contamination and irradiation. They are helpful on their own, though, and extremely different from one another.
Applications of nuclear radiation
Sterilization using radiation
Fruit sold in supermarkets can be preserved through radiation by subjecting it to a radioactive source, usually cobalt-60. .
Any bacteria on the fruit will be destroyed by the cobalt's gamma rays, but the fruit won't undergo any appreciable changes.
The irradiated object does not become radioactive during the irradiation process.
Medical irradiation
Physicians employ radioactive sources for many purposes, such as:
sterilizing surgical equipment.
Gamma-ray beams can be utilized to destroy malignant tumors located deep within the body. To maximize the dose on the tumor and minimize the dose on the surrounding soft tissue, the beams are directed in a variety of directions towards the tumor.
Since this method can harm good tissue, great care is taken to choose the ideal dosage—enough to destroy the tumor, but not enough to cause harm to the surrounding tissue.
A particular kind of gamma irradiation called a "gamma knife" is used to destroy malignant brain cells. The head is secured in a specialized helmet to ensure that it remains still throughout the radiation treatment.
When employing radioactive sources in medical applications, precautions are taken to guarantee that radiation exposure has no long-term effects. To do this, take into account:
the type of decay (beta, gamma, or alpha)
the half-life, which is both sufficiently long for the isotope to yield valuable measurements and sufficiently brief for the radioactive sources to quickly decay to safe levels after usage.
toxicity
The radiation's harmful effects would endure too long and the dose would keep rising if the half-life selected was too lengthy.
Advantages and disadvantages of radiation irradiation
Advantages
It is possible to sterilize without using very hot temperatures.
It can be applied to objects that would melt to eliminate microorganisms.
Disadvantages
Not every bacteria on an object will be eliminated.
Standing near items that are being treated with radiation can be extremely dangerous as it can cause damage and mutation to people's cells.
Medical contamination
In certain instances, radioactive sources that are injected (such technetium-99) can be utilized as tracers to make soft tissues visible during medical imaging procedures, such as the kidneys or blood vessels.
Gamma rays released by an isotope can readily penetrate the body and reach an external detector, such as an X-ray machine or "gamma camera."
This makes it possible to track the radioactive isotope as it moves through a specific bodily activity. Variations in the gamma radiation output from various components would reveal the state of the isotope flow or the presence of any obstructions.
In medical applications when injectable radioactive sources are used, precautions are taken to guarantee that contamination won't have any lasting effects. To accomplish this, select isotopes that:
has extremely brief half-lives; the sources that are employed usually have half-lives of hours. As a result, after a few days, a person's body will rarely contain any radioactive material at all.
are not toxic
Contamination in order to detect leaks
To locate pipe leaks, radioactive isotopes that generate gamma rays can be introduced into water sources.
Gamma emissions accumulate in the vicinity of a leak because tainted water penetrates into the earth.
The accumulation of radioactive emissions can be determined with a Geiger-Müller tube. This facilitates the decision of where to dig to locate the leak.
The isotope utilized in this process needs to:
possess gamma radiation
possess a minimum half-life of a few days to enable the emissions to accumulate in the soil.
as it will be a component of the water supply, it won't be toxic to people.
Radiation sickness
Materials that are radioactive are dangerous. Chemicals in the body can become ionized by nuclear radiation, altering the behavior of the cells.
Moreover, it has the capacity to absorb massive quantities of energy into the body, which can totally destroy or harm cells.
Eyes
Cataracts may develop from high dosages.
Thyroid
Especially during growth, radioactive iodine can accumulate and cause cancer.
Lungs
Inhaling radioactive particles can harm DNA.
Stomach
The stomach is susceptible to long-term radiation exposure from radioactive isotopes.
Reproductive organs
Excessive dosages may result in mutations or sterility.
Skin
Radiation can result in cancer or burn the skin.
Bone marrow
Leukemia and other blood disorders can be brought on by radiation.
Managing risks
The degree of exposure to radioactive elements determines the danger.
The dosage acquired by exposure to highly radioactive materials, radioactive materials for extended periods of time, or radioactive materials on a regular basis increases, increasing the danger.
Using radioactive sources can be made less risky by taking specific measures, since radioactive elements are harmful. Among them are:
When not in use, keep radioactive sources like technetium-99 protected (ideally in a lead-lined box).
Put on protective gear in case radioactive isotopes spill and poison the body.
Do not touch exposed skin, and do not try to taste the sources. Put on face masks to prevent inhaling harmful substances.
restrict the amount of time spent in close proximity to radioactive materials by handling them with tongs and limiting exposure duration to maintain a safer distance from sources.
use detector badges, etc., to track exposure
The kind of radiation to which a person has been exposed, as well as whether that exposure was internal or external, also affect the risk.
In the event that the radioactive source enters the body by ingestion or inhalation:
The most hazardous type of radiation is alpha because cells may readily absorb it.
Because beta and gamma radiation are more likely to flow through cells than be absorbed, they are not as harmful.
If the source of radiation is external to the body:
Because alpha radiation is absorbed by the skin and is unlikely to reach living cells within the body, it is not extremely hazardous.
Nuclear fission
The division of a massive atomic nucleus into smaller nuclei is known as nuclear fission.
A neutron is absorbed into a nucleus (usually uranium-235) in a nuclear reactor. As a result, the nucleus turns into the very unstable uranium-236. Two sizable pieces known as "daughter nuclei" are formed when the complete nucleus divides.
Apart from the 'daughter' products, the fission reaction also releases two or three neutrons, which can collide with other uranium nuclei to initiate more fission reactions.
It is called a chain reaction. Gamma radiation and the kinetic energy of the particles are two ways that energy is released from the nucleus. The majority of this energy is used to heat water for the generators' turbines.
Similar to how energy is released from a chemical store, like explosives, after an explosion, but much more, is the release of energy from the nuclear energy storage, uranium.
Nuclear fusion
The process by which two tiny, light nuclei combine to form one large nucleus is known as nuclear fusion.
In stars, fusion processes take place when two hydrogen nuclei combine at extreme pressure and temperature to create the nucleus of an isotope of helium.
The Sun is experiencing several distinct nuclear fusion processes. When four hydrogen nuclei combine to form one helium nucleus, it is the most basic:
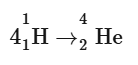
Four hydrogen nuclei have a combined mass of 6.693 × 10-27 kg. A single helium nucleus has a mass of 6.645 × 10-27 kg.
This indicates that 0.048 x 10-27 kg of mass is lacking.
Energy is created from the missing mass and radiates outward. This is observed taking place in the Sun. Atomic reactions always involve a modest conversion of mass into energy.
The problem with fusion, though, is that it necessitates the merging of positive particles called nuclei. Due to their identical charges, two nuclei will resist one another as they get closer. In order to prevent the repulsion of the charges from stopping the fusing of the nuclei, it must occur swiftly.
Particles can move very swiftly when they are in a heated gas or in plasma like in the Sun.