physics eoy - y9
key words
accuracy - result that is close to true value
interval - quantity between readings
precision - all the results are very close to eachother
range - minimum and maximum values
resolution - smallest change in the quantity being measured
repeatable - get the same result with same method
reproducible - get the same results with a different method / someone else doing it
uncertainty - interval within which the true interval can lie
measurement error - measure something wrong
random error - reading is different every time
systematic error - a constant is off, getting the same reading each time but it’s wrong
zero error - reading doesnt start at 0
conductor - material that allows heat energy to move through
insulator - material in which heat energy doesnt move freely
chapter 2 - energy transfer by heating
2.1 energy transfer by conduction
metals conduct energy better than non metals
copper is a better conductor than steel
glass conducts better than wood
the energy transfer by conduction through a material depends on it’s thermal conductivity
the greater the thermal conductivity, the more energy per second it transfers via conduction
good insulators have low thermal conductivity, so that there is as little energy transfer through them
the more the atoms vibrate, the hotter the substance is
energy transfer per second depends on:
temp difference across the material
thickness of the material
thermal conductivity of the material
2.2 + 2.3 infrared radiation (IR)
the higher the temperature, the more IR radiation it emits
all objects absorb and emit IR radiation
a perfect black body absorbs all radiation it is hit with
an object which has a constant temperature emits radiation across a continuous range of wavelengths
the temperature of an object increases when it absorbs more radiation than it emits, and vice versa
the earths temperature depends on things like absorption of IR radiation from the sun, and the emission of radiation from the earths surface and atmosphere
if an object absorbs and emits the same amount of energy, it remains the same temperature because it is gaining and losing the same amount of heat
intensity - how much energy the radiation transfers to a given area in a set amount of time
as the temperature increases:
the intensity of every emitted wavelength increases
the intensity of shorter wavelengths increases
for objects at room temperature, the emitted radiation is all in the IR range, rather than visible light, like the Sun
during the day, the earth absorbs more IR than it emits, so it’s local temperature increases
during the night, the earth absorbs less IR than it emits, so it’s local temperature decreases
overall, some part of the earth is always in the sun, so the overall temperature remains constant
2.4 specific heat capacity (shc)
the temperature of an object rises depending on:
amount of energy supplied to it
mass
what the substance is
specific heat capacity is how much heat energy in Joules is required to raise the temperature of 1kg of a substance by 1 degree Celsius
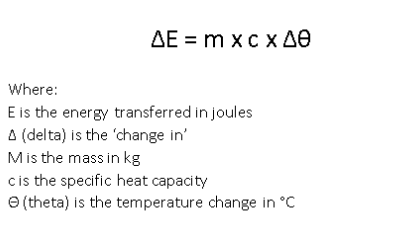
the greater the mass, the slower it increases in temperature
high SHC - more energy required
heat - energy required for the temperature to rise in Joules
temperature - measuring kinetic energy of the particles in degrees Celsius
storage heaters use electricity at night to heat special bricks/concrete through energy transfer, as bricks have a high SHC so they store lots of energy, and heat up and cool down slowly
2.5 heating and insulating buildings
LOFT INSULATION
eg. fibreglass
reduces energy transfer rate in roof
more insulation layers, thicker insulation, keeps it more warm
CAVITY WALL INSULATION
reduces energy transfer rate
traps air in small air pockets
made of foam
insulation material used to fill the cavity between the two brick layers of an external house wall
DOUBLE GLAZED WINDOWS
2 glass panes with dry air/a vacuum between the panes
dry air is a good insulator
thick glass slows the rate of transfer of energy
EXTERNAL WALL
thicker bricks means less heat will escape from the home
lowers electrical costs
SOLAR PANELS
absorb infrared radiation from the sun
generates electricity directly
also helpful - close fitting door and carpets on floor with rubber underlay
chapter 6 - molecules and matter
6.1 density
density is the mass per unit volume
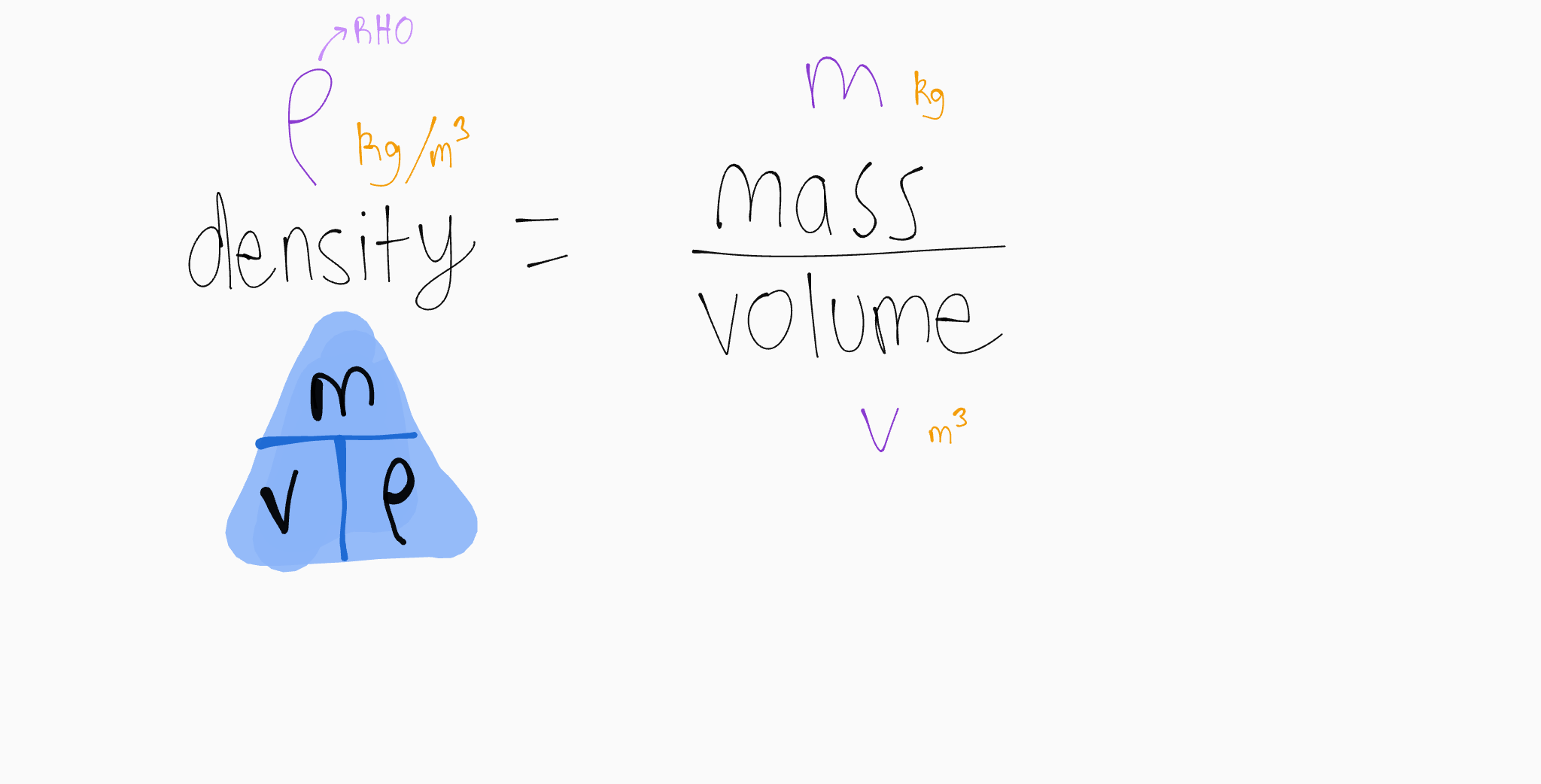
volume of a sphere = 4 x pi x radius³ / 3
objects with a lower density than water (<100kg/m³) will float in water
6.2 states of matter
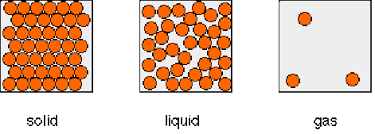
STATE | FLOW | SHAPE | VOLUME | DENSITY | FORCES OF ATTRACTION | ENERGY |
|---|---|---|---|---|---|---|
SOLID | no | fixed | fixed | higher than gas | strong | less than liquid and gas |
LIQUID | yes | fits container shape | fixed | higher than gas | medium | more than solid, less than gas |
GAS | yes | fills container | changeable | less than solid or liquid | weak | more than solid or liquid |
solid to liquid - melting
liquid to solid - freezing
gas to liquid - condensation
liquid to gas - vaporisation / boiling
solid to gas - sublimation
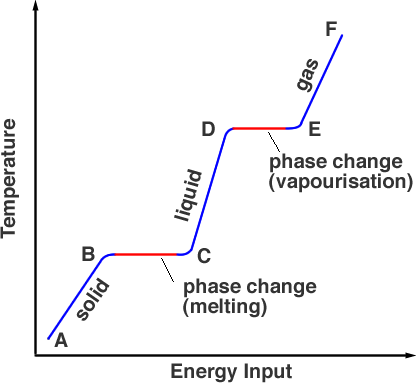
6.3 changes of state
temperature stays the same when changing state
energy transferred to a substance when it changes state is called latent heat
energy is needed to melt a solid or boil a liquid
the flat section of a temp-time graph shows the melting/boiling point of a substance
6.4 internal energy
energy stored by the particles of a substance is called the substances INTERNAL ENERGY
internal energy is the sum of:
kinetic energy they have due to their individual motions, relative to eachother
potential energy they have due to their individual positions relative to each other
therefore the internal energy of a substance is:
the total energy in the kinetic and potential energy stores of the particles in the substance that is caused by their individual motions and positions
increase temp, increase internal energy
the pressure of a gas on a surface is caused by the particles of the gas repeatedly hitting the surface
6.5 specific latent heat (slh)
SLH OF VAPORISATION OR FUSION IS THE ENERGY NEEDED TO MELT OR BOIL 1KG OF A SUBSTANCE WITHOUT CHANGING IT’S TEMPERATURE
fusion - solid to liquid
vaporisation - liquid to gas
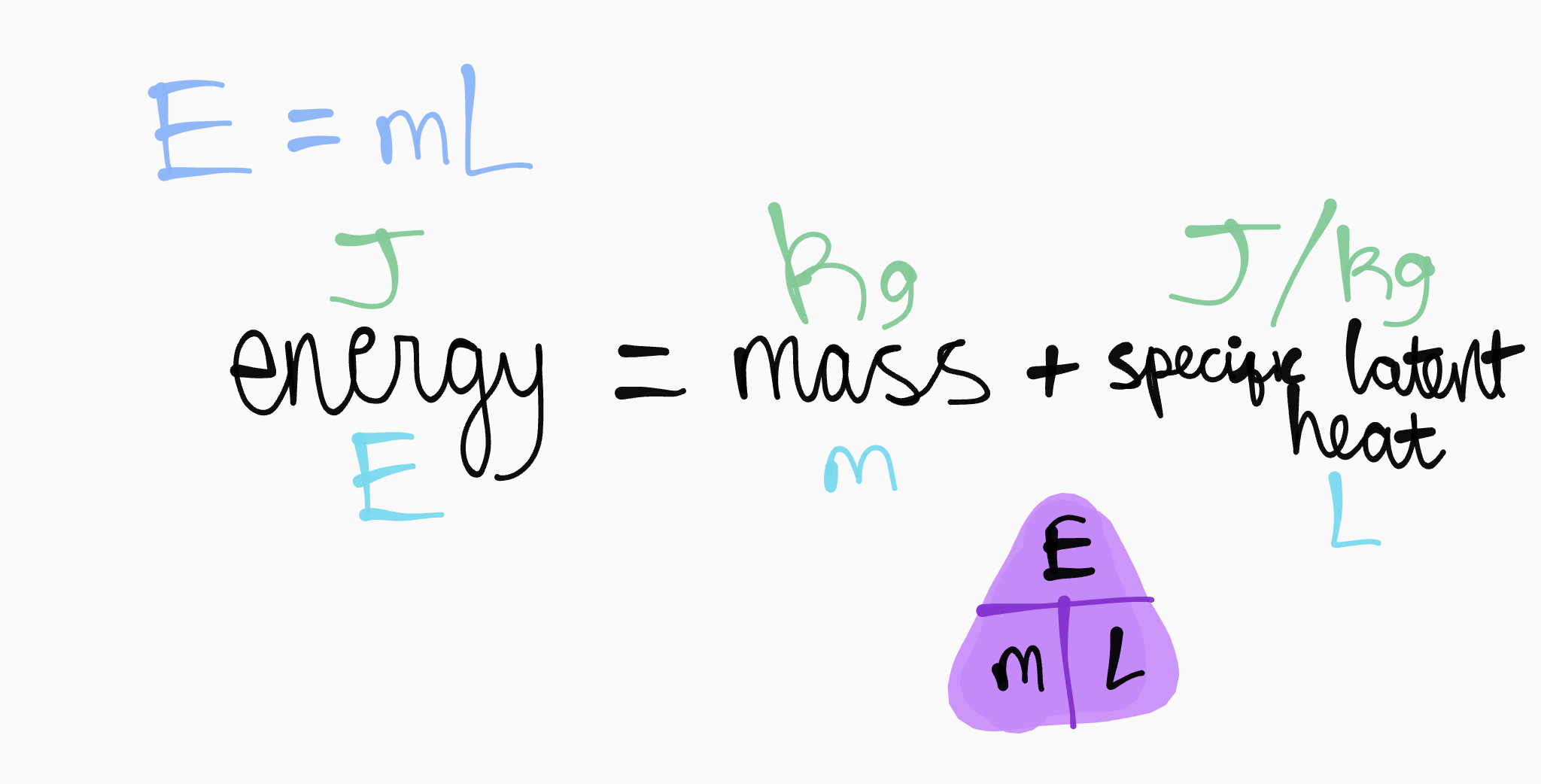
6.6 gas pressure and temperature
increasing the temperature of any sealed gas container increases the pressure of the gas inside it
pressure is proportional to temperature
6.7 gas pressure and volume
when a gas is:
COMPRESSED, it decreases in volume and increases in pressure
EXPANDED, it increases in volume and decreases in pressure
for a fixed mass of gas, the no. of gas molecules is constant
if the temperature is constant, the average speed of the molecules is constant
pressure and volume are inversely proportional, so when the pressure increases, the volume decreases, and vice versa
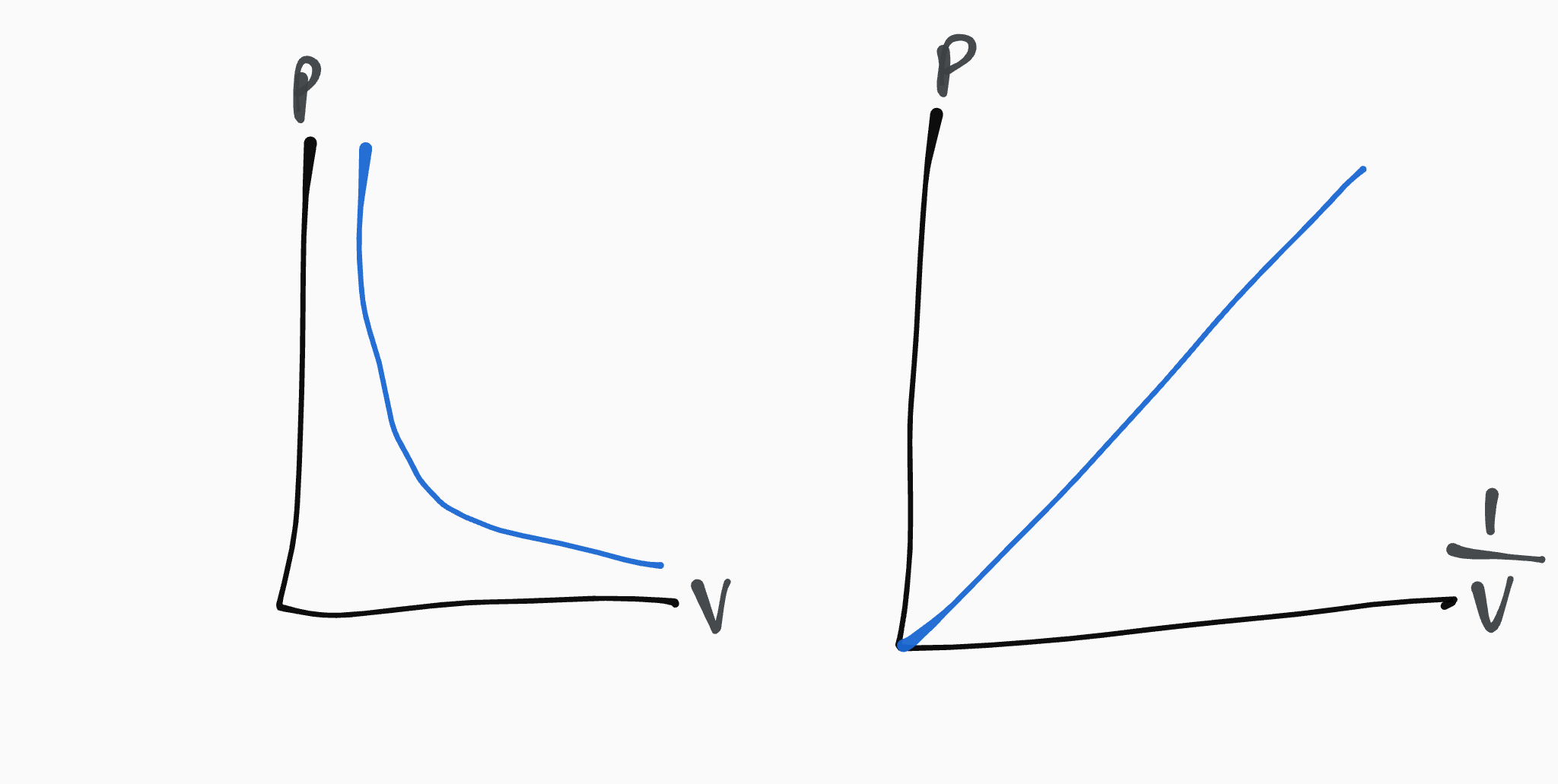
BOYLES LAW
for a fixed mass of a gas held at constant temperature,
pV = constant
P1V1 = P2V2
P = pressure, Pascals (Pa)
V = volume, metres cubed (m³)