Cell Membrane and Transport Notes
The Plasma Membrane: The Gatekeeper of the Cell
Learning Objectives
Describe the structure of cell membranes.
Identify hydrophilic and hydrophobic regions.
Describe the roles of phospholipids, proteins, and carbohydrates in membranes.
Explain the significance of the semi-permeable membrane.
Cell Membrane Structure and Function
The plasma membrane defines the cell's boundary and mediates interaction with the environment. It controls the movement of substances in and out of the cell.
Flexibility: The membrane is dynamic and flexible, allowing cells like red and white blood cells to change shape when passing through capillaries.
Recognition: Surface markers enable cell recognition, crucial for tissue and organ formation and immune response.
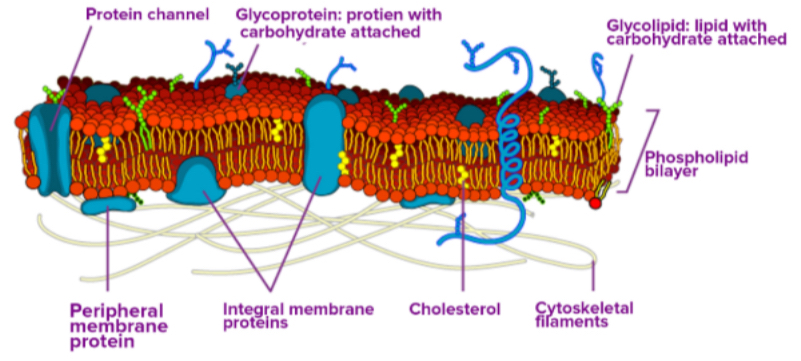
Components of the Plasma Membrane
The plasma membrane consists of a double layer of phospholipids with embedded proteins and carbohydrates.
Phospholipids: Form the main structure with hydrophilic heads and hydrophobic tails.
Proteins: Embedded (integral) or attached (peripheral) proteins serve as channels, pumps, enzymes, structural attachments, or recognition sites.
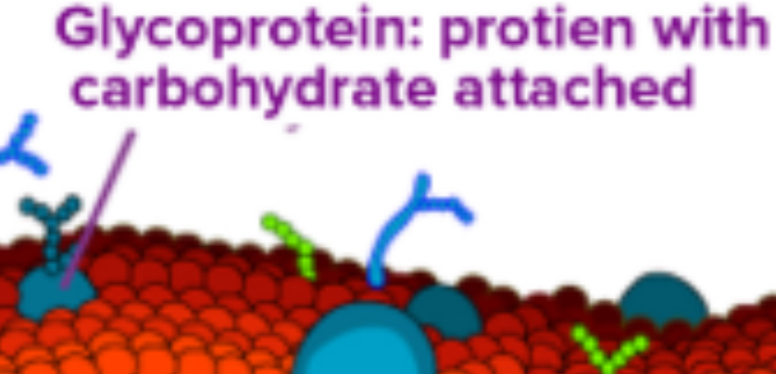
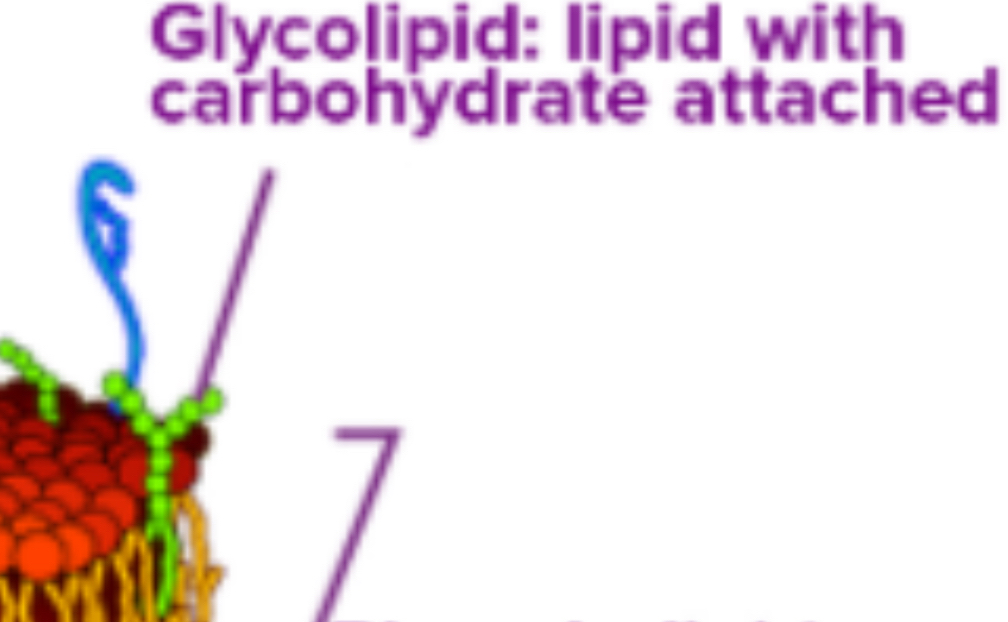
Carbohydrates: Located on the exterior surface, bound to proteins (glycoproteins) or lipids (glycolipids), and facilitate cell recognition.
Receptors
Receptors on the plasma membrane are attachment sites for specific substances.
Specificity: Each receptor binds to a specific substance, triggering changes within the cell (e.g., altering enzymes in metabolic pathways).
External Interactions: Receptors interact with hormones or neurotransmitters, transmitting their messages into the cell.
Viral Exploitation: Viruses can exploit recognition sites to enter cells by mimicking specific substances. Hence why HIV binds to only specific cells
Fluid Mosaic Model
The fluid mosaic model describes the plasma membrane as a dynamic mosaic of phospholipids, cholesterol, proteins, and carbohydrates that can move and change position while maintaining membrane integrity.
Fluidity: Phospholipids and embedded proteins can diffuse rapidly and laterally within the membrane. Membrane fluidity is essential for enzyme activity and transport molecule function.
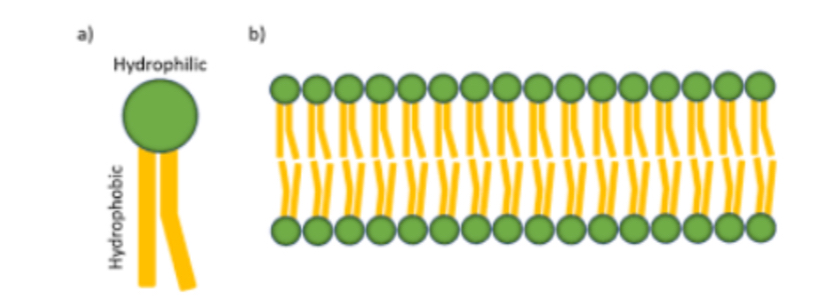
Hydrophilic and Hydrophobic Regions
The membrane consists of two layers of phospholipid molecules.
Hydrophilic Surfaces: The polar (hydrophilic) ends of phospholipids face the aqueous fluid inside and outside the cell.
Hydrophobic Interior: The interior of the membrane is hydrophobic due to the fatty acid tails.
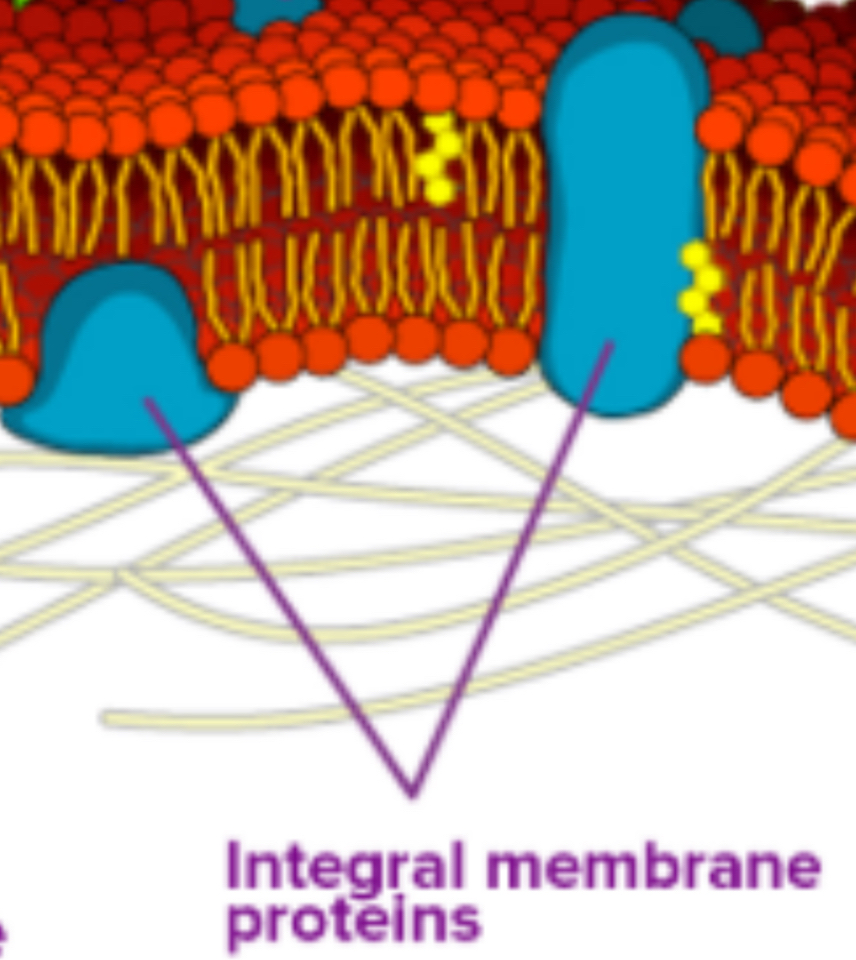
Proteins in the Membrane
Proteins are a major component of plasma membranes.
Integral Proteins: Embedded in the membrane, spanning all or part of it. They act as channels or pumps for moving materials in and out of the cell.
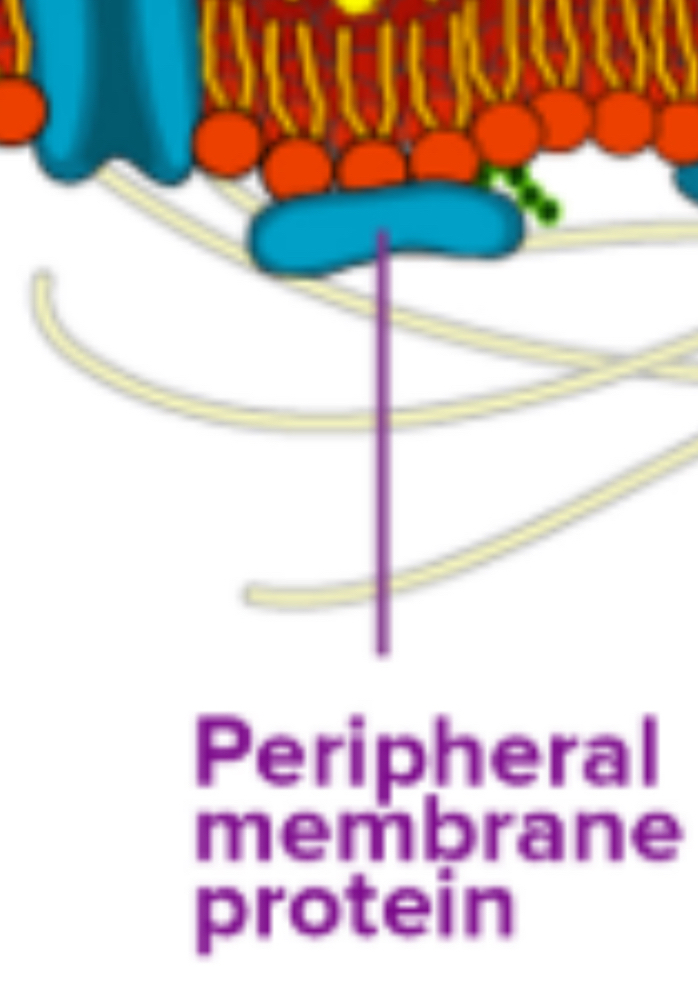
Peripheral Proteins: Located on the exterior or interior surfaces, attached to integral proteins or phospholipids. They function as enzymes, structural attachments, or recognition sites.
Carbohydrates in the Membrane
Carbohydrates are always found on the exterior surface and are bound to proteins (glycoproteins) or lipids (glycolipids).
Recognition Sites: Along with peripheral proteins, carbohydrates form specialized sites for cell recognition.
Cholesterol in the Membrane
Cholesterol is embedded in the membrane and regulates fluidity.
Fluidity Regulation: The amount of cholesterol affects membrane fluidity based on temperature. It acts as an antifreeze, more abundant in animals in cold climates.
Membrane Transport
The plasma membrane regulates the movement of molecules and ions in and out of the cell.
Selective Permeability: The membrane is semi-permeable, allowing some materials to cross while excluding others.
Glucose Transport: Glucose requires special transport proteins (GLUTs) to move across the membrane.
Passive Transport
Learning Objectives
Explain why and how passive transport occurs.
Define osmosis and diffusion.
Define tonicity and describe its relevance to membrane transport.
Selective Permeability
Plasma membranes are selectively permeable, allowing specific substances to pass through while preventing others.
Passive vs. Active Transport: Some materials move passively (without energy), while others require energy (active transport).
Concentration Gradient: The uneven distribution of molecules or ions across the membrane is called a concentration gradient, essential for cell function.
Concentration gradient: high concentration to low concentration or low concentration to high concentration
Diffusion
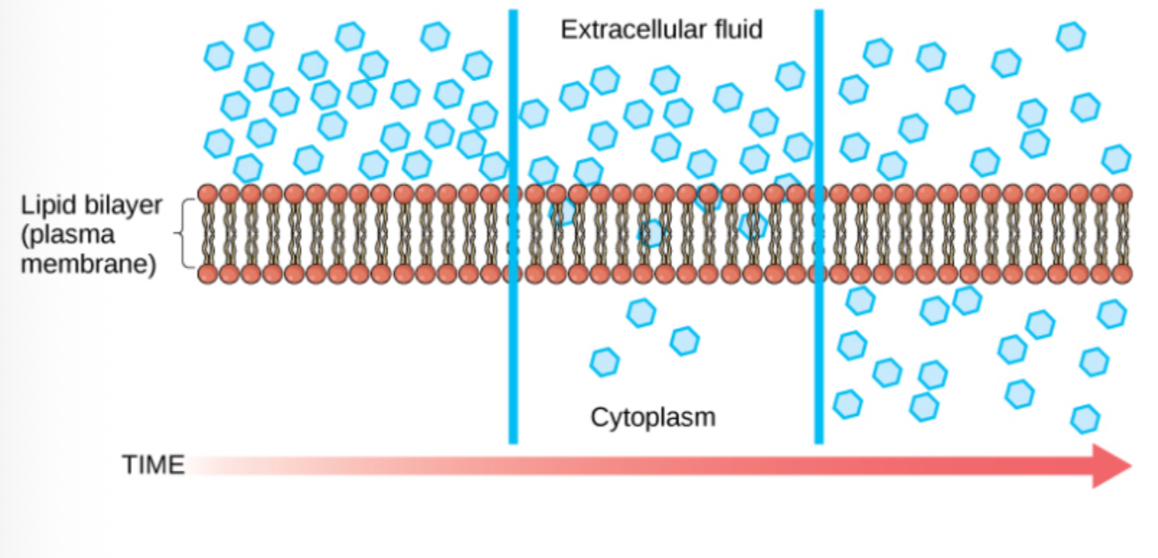
In passive transport, substances move from an area of higher concentration to lower concentration.
Active Transport: REQUIRES ENERGY to move substances against their concentration gradient.
Plasma membranes are asymmetric; the interior differs from the exterior.
Integral Proteins and Carbohydrates: Found on the exterior surface, facilitating binding of needed substances.
Lipid-soluble materials easily pass through the hydrophobic lipid core.
Fat-Soluble Vitamins and Drugs: Readily pass through membranes.
Gases: Oxygen and carbon dioxide pass through via simple diffusion.
Polar Substances
Most polar substances face difficulty passing through the membrane.
Ions, Sugars, and Amino Acids: Require special transport mechanisms to penetrate plasma membranes.
Diffusion
Diffusion is a passive process where a substance moves from high to low concentration until equilibrium is reached.
Factors Affecting Diffusion: Concentration difference, molecule size, and temperature affect the rate of diffusion.
Facilitated Diffusion
Facilitated transport uses transmembrane proteins to move materials down a concentration gradient WITHOUT energy expenditure.
Transport Proteins: Integral proteins act as channels or carriers for specific substances.
PROTEINS USED TO TRANSPORT MATERIALS WITHOUT ENERGY
Osmosis
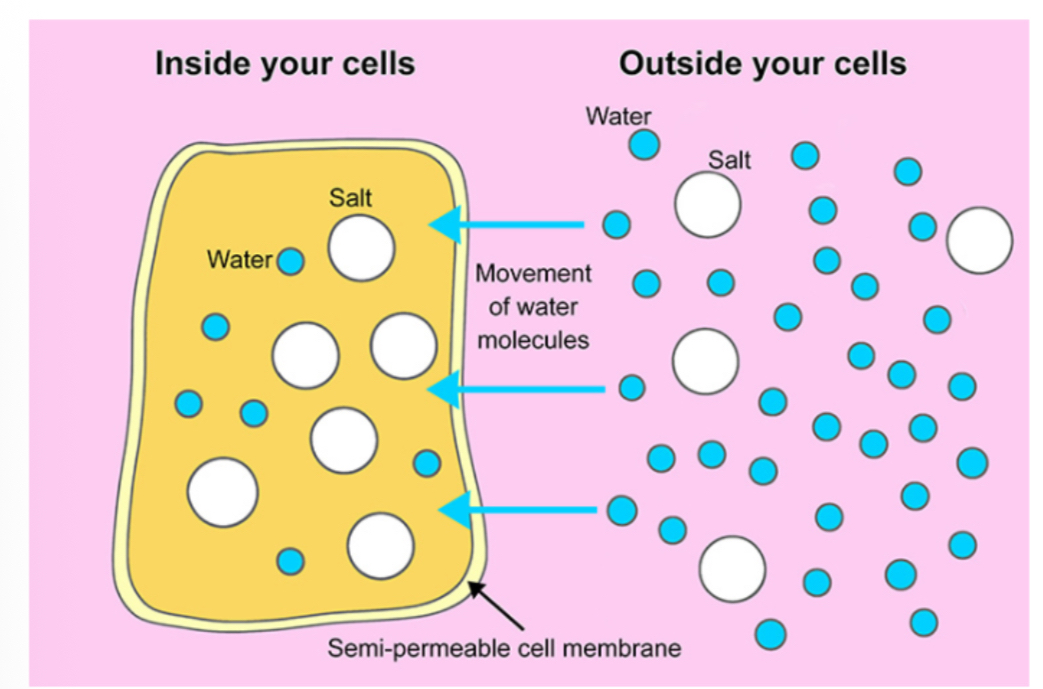
Osmosis is the movement of water through a semipermeable membrane according to its concentration gradient.
Water Movement: Water moves from high to low concentration.
Solute Concentration: Water concentration is inversely proportional to solute concentration.
Tonicity
Tonicity describes the solute concentration of a solution.
Osmolarity: The measure of tonicity.
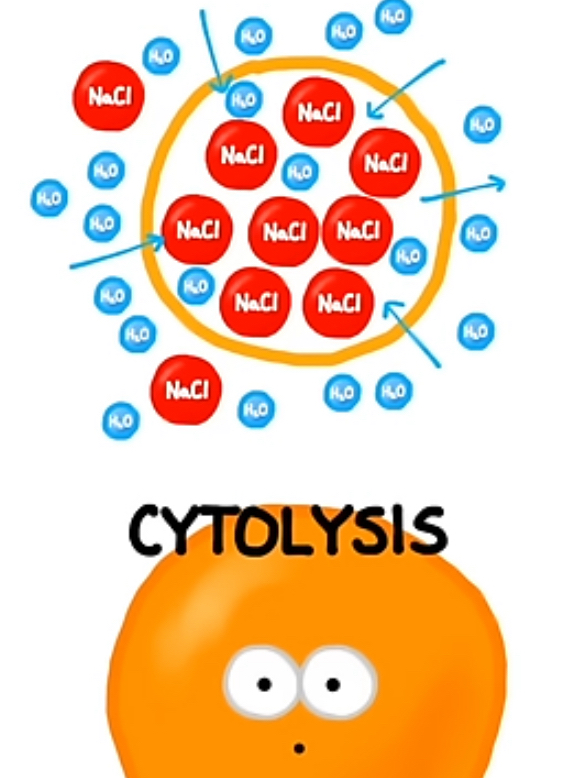
Hypotonic: The extracellular fluid has a lower solute concentration than the cell, causing water to enter the cell and potentially lyse it.
Outside has little solute and high water, Inside of cell has high solute and low water. Solute cannot move so what must move is the water and it will move from high to low concentration so outside to inside of cell
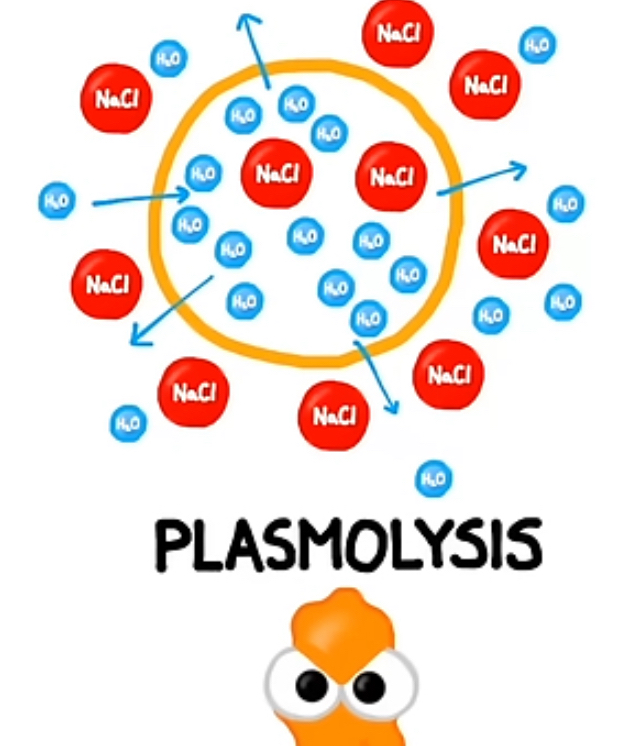
Hypertonic: The extracellular fluid has a higher solute concentration, causing water to leave the cell and potentially crenate it.
Outside has high solute and little water and inside has little solute and high water. Causing water to move out because inside has high and outside has low concentration
Isotonic: The extracellular fluid has the same osmolarity as the cell, resulting in no net water movement.
Cell Walls: Prevent cell lysis in organisms like plants, fungi, and bacteria.
Active Transport
Learning Objectives
Define a concentration gradient.
Explain how cells use chemical gradients.
Explain why some molecules require energy to move across the membrane.
Describe bulk transport, including endocytosis (phagocytosis, pinocytosis and receptor-mediated endocytosis) and exocytosis.
Active transport uses cellular energy, usually in the form of ATP.
Against the Gradient: Moves substances against their concentration gradient.
Larger Molecules: Requires energy for uptake and release.
Electrochemical Gradient
Gradients are complex in living systems due to charged proteins and ion movement.
Electrical Gradient: The interior of cells is electrically negative compared to the extracellular fluid.
Ion Concentrations: Cells have higher K+ and lower Na+ concentrations than the extracellular fluid.
Electrochemical Gradient Definition: The combined gradient affecting an ion is crucial for muscle and nerve cells.
Moving Against a Gradient
Active transport mechanisms use energy from ATP to move substances against concentration gradients.
Pumps or Carrier Proteins: Work against electrochemical gradients.
Metabolic Poisons: Active transport is sensitive to metabolic poisons that interfere with ATP supply.
Primary and Secondary Active Transport
Primary Active Transport: Uses ATP to move ions across a membrane, creating a charge difference. An example is the sodium-potassium pump.
3Na^+ ions are transported out of the cell, while 2K^+ ions are transported into the cell per ATP molecule hydrolyzed.
This process maintains a negative charge inside the cell contributing to the resting membrane potential.
Secondary Active Transport: Uses the electrochemical gradient created by primary active transport to move other substances such as amino acids and glucose.
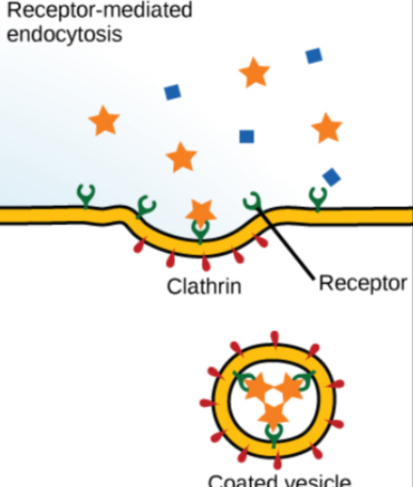
Endocytosis
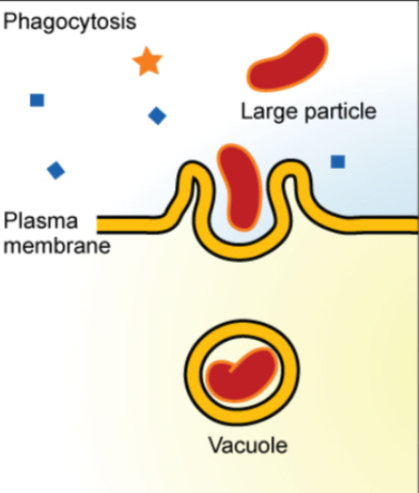
Endocytosis moves large particles into a cell.
Mechanism: The plasma membrane invaginates, forming a pocket around the target particle, which pinches off into a vacuole.
Phagocytosis: The cell membrane surrounds and engulfs large particles (e.g., microorganisms).
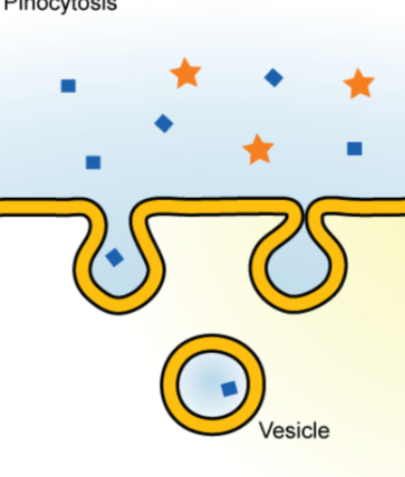
Pinocytosis: The cell membrane surrounds and engulfs small volumes of fluid. Because it wants the solute particles.
Receptor-Mediated Endocytosis: Uptake is targeted to specific substances that bind to receptors on the cell membrane.
Exocytosis
Exocytosis expels material from the cell into the extracellular fluid.
Mechanism: A particle enveloped in membrane fuses with the plasma membrane, releasing the contents outside the cell.
Lesson 2 Slides Notes
Learning Objectives
Explain the fundamentals of atomic structures and define a bond.
Explain the main function of a cell's plasma membrane.
Describe the functions of phospholipids & transport proteins in the plasma membrane
Identify the hydrophobic & hydrophilic regions of the phospholipid bilayer.
List the kinds of molecules that can & cannot diffuse across the membrane.
Use the chemical properties of a molecule (hydrophobic, hydrophilic, polar, nonpolar, charged) to determine whether or not it could diffuse across the membrane.
Explain what a concentration gradient is.
Use the direction of a concentration gradient to determine which way a molecule would move (into or out of a cell).
Explain how passive transport is different from active transport.
Explain why active transport is important to a cell.
Identify similarities & differences between these transport processes: diffusion, facilitated diffusion osmosis, primary active transport, secondary active transport.
Atoms and Chemical Bonds
Atom: A particle of matter consisting of a nucleus surrounded by electrons.
Chemical Bond: A lasting attraction between atoms that enables the formation of molecules. Breaking bonds releases energy, while forming new bonds absorbs and stores energy.
ATP Structure
ATP (Adenosine Triphosphate) contains high-energy chemical bonds. The molecule with more chemical bonds has more energy.
ATP is used in reactions by breaking the bond between the last two phosphate groups, releasing energy.
Cells & the Plasma Membrane
Cell Number: The human body is made of approximately 30 trillion cells.
Plasma Membrane Function: Divides the inside of a cell from its environment.
Cytosol: Intracellular fluid found inside the cell.
Extracellular Fluid: Fluid found in the environment outside the cell.
Selective Permeability: Only certain molecules can pass directly through the plasma membrane, based on its structure.
The Phospholipid Bilayer
Structure: The plasma membrane is composed of two rows of phospholipids.
Components: Each phospholipid has a hydrophilic head group and two hydrophobic fatty acid tails.
Polarity of Water
Water Composition: Water consists of two hydrogen atoms and one oxygen atom.
Electron Sharing: Electrons are not shared equally, giving oxygen a partial negative charge and hydrogen a partial positive charge.
Polarity: Water is considered a polar molecule due to these partial charges.
Selective Permeability
Only molecules that match the chemical properties of the fatty acid tails can diffuse across the membrane.
Matching Molecules: Hydrophobic molecules (like steroid hormones) and nonpolar gases can diffuse.
Polar Molecules: Small polar molecules can sometimes squeeze between fatty acid tails, but large ones cannot.
Transport Proteins
Transport proteins are required for most substances that do not match the fatty acid tails.
Examples: Glucose, amino acids, and ions require transport proteins.
Membrane Permeability Summary
The direction of the arrow determines whether a molecule can diffuse straight across the plasma membrane (blue) or whether a transport protein is required (black).
Passive Transport Types
Passive transport does not require energy and includes diffusion, facilitated diffusion, and osmosis.
Diffusion (Simple Diffusion): Molecules travel directly through the plasma membrane (hydrophobic and nonpolar molecules).
Facilitated Diffusion: Molecules use transport proteins to travel through the plasma membrane (hydrophilic, polar, and large molecules).
Osmosis: Water travels through the plasma membrane (a specific type of facilitated diffusion using aquaporins).
Concentrations
Fluids of the body are solutions (liquids with dissolved solutes).
Solvent: The liquid part of a solution (in the body, it's water).
Solutes: The dissolved substances in a solution.
Concentration: Represents the number of solutes dissolved in a solution (high concentration = lots of solutes; low concentration = fewer solutes).
Concentration Gradients & Movement
Concentration Gradient: A difference in concentrations between two solutions.
Movement Direction: Molecules naturally move down their concentration gradient (from high to low concentration), which does not require energy (passive transport).
Osmosis & the Human Body
Water constantly moves into and out of cells based on the concentration gradient between the cytosol and other body fluids.
Hypertonic: The solution has a higher solute concentration than the cytosol.
Isotonic: The solution has the same solute concentration as the cytosol.
Hypotonic: The solution has a lower solute concentration than the cytosol.
Passive Transport: A Review
Does not require energy.
Moves molecules (solutes or solvents) from areas of high concentration to areas of low concentration.
Diffusion: Molecules move directly across the plasma membrane.
Facilitated Diffusion: Molecules use a transport protein to move across the plasma membrane.
Osmosis: Water moves across the membrane.
Active Transport
Sometimes a cell needs to move a molecule UP (against) its concentration gradient (From low to high concentration).
This requires energy from:
ATP: Stores energy in its chemical bonds.
A concentration gradient: As one molecule moves down its concentration gradient, that movement pushes a different molecule up its own gradient.
Primary Active Transport
Uses ATP as energy source.
Example: The sodium-potassium pump
Secondary Active Transport
Movement down one concentration gradient (Na+) is used to power movement up a different concentration gradient (glucose).