9.1 Acids and Bases:
Acids Produce Hydronium Ions and Bases produce Hydroxide Ions in Aq Solutions:
Arrhenius definition:
Acid: produces H3O or hydronium ions. polyatomic ion w/ 3 O-H bonds, has a charge of +1.
Bases: hydroxide ion, OH- in a aq solution. They undergo a solvation in water
` Ionic Compounds:
electrolytes
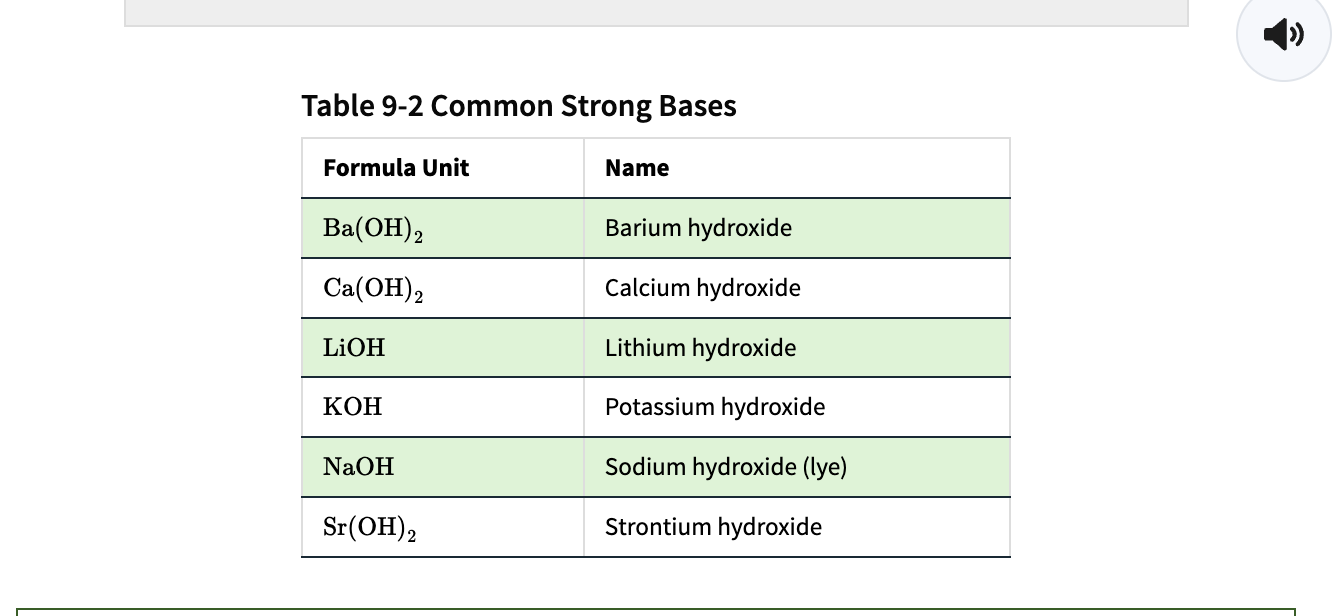
Group 1A salts of hydroxide = strong electrolytes & they are soluble in water.
` Ionic Compounds w/ hydroxide ions
Ex: Mg(OH)2 - magnesium hydroxide
a base due to producing hydroxide ions in solution
weak electrolytes
The Bronsted-Lowry Theory of Acids and Bases:
Bronsted-Lowry Theory:
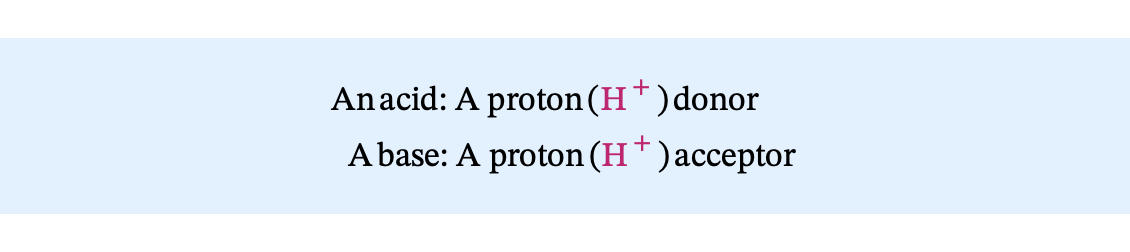
an acid: donates a proton, H+
a base: accepts a proton from an acid.
Acids:
This is according to Bronsted-Lowry:
water has been added to an acid.
acid will donate a proton to water = this will for a conjugate base
water accepts a proton = forms a hydronium ion
hydronium ion = the conjugate acid of water.
Conjugate Base: ion or molecule produced when an acid loses a proton
Bases:
This is according to Bronsted-Lowry Theory:
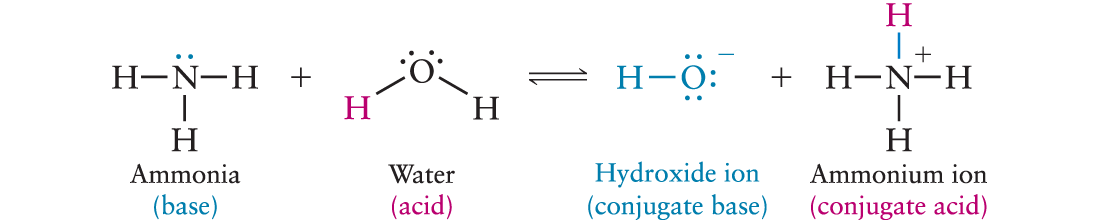
a base gets water added = a proton from water will be accepted.
water is the acid in this scenario
base + water = hydroxide ion OH- and ammonium ion NH4+.
Conjugate Acid: ion/molecule formed when a base accepts a proton.
Amphoteric Compounds: compounds that can become either an acid or a base.

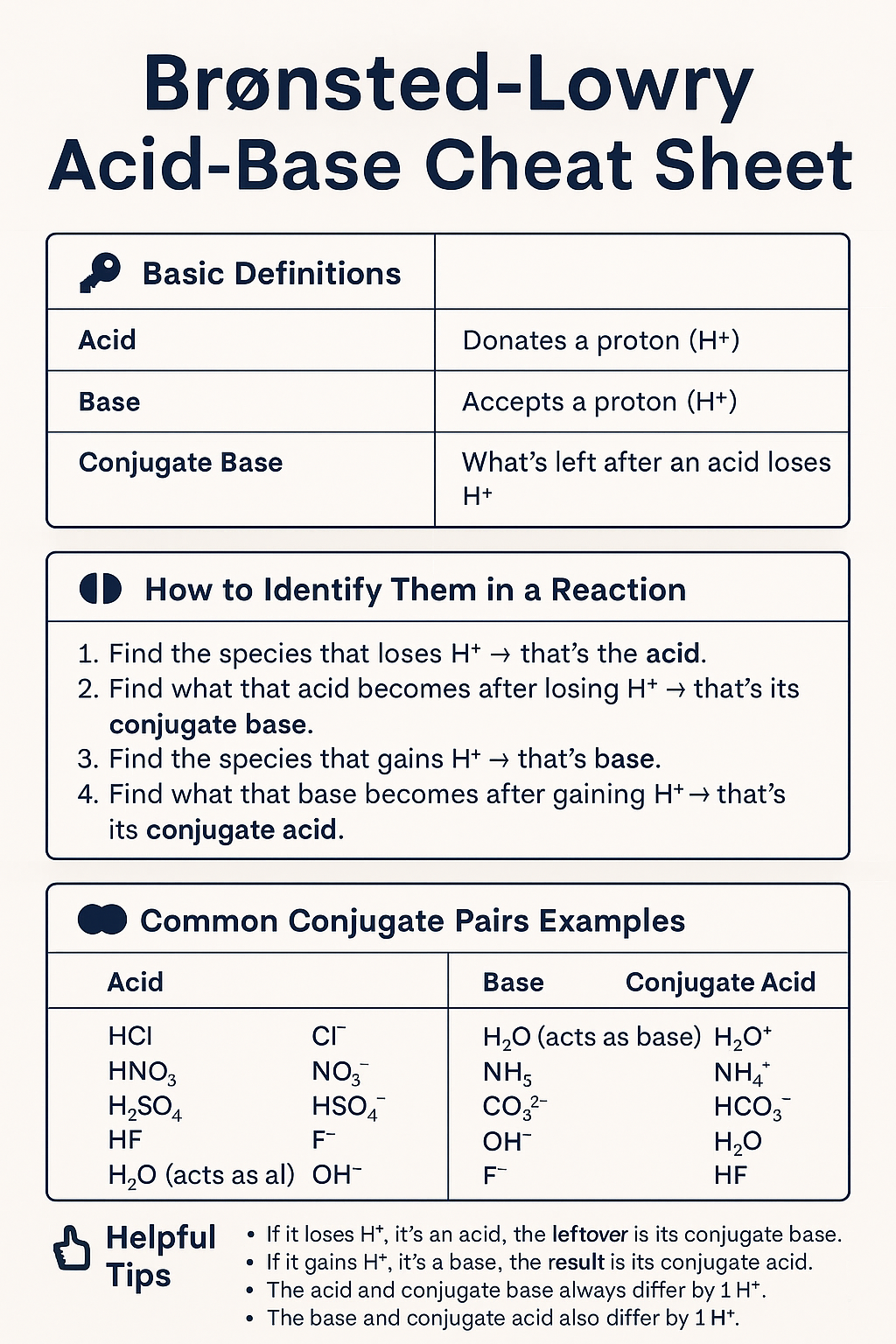
Conjugate Acid-Base Pairs:
Conjugate acid base pair: can be the pair of
base and its conjugate base
acid and its conjugate base
determining the structure/formula of conjugate bases:
Difference between acid-base pairs:
conjugate acid of a base: has 1 more hydrogen, and charge one unit more positive than the base.
Term | What it Means |
|---|---|
Acid | Donates a proton (H⁺) |
Base | Accepts a proton (H⁺) |
Conjugate Base | What’s left after an acid loses H⁺ |
Conjugate Acid | What’s formed when a base gains H⁺ |
How to Identify Them in a Reaction
Find the species that loses H⁺ → that’s the acid.
Find what that acid becomes after losing H⁺ → that’s its conjugate base.
Find the species that gains H⁺ → that’s the base.
Find what that base becomes after gaining H⁺ → that’s its conjugate acid.
Common Conjugate Pairs Examples
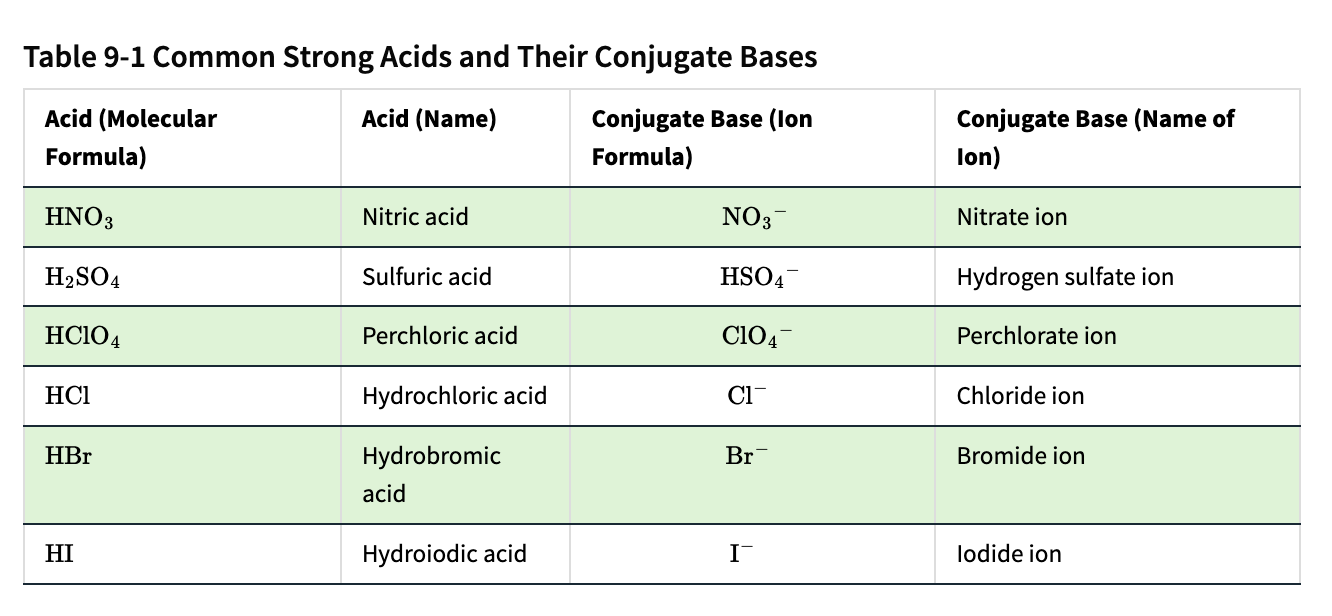
Acid | Conjugate Base |
|---|---|
HCl | Cl⁻ |
HNO₃ | NO₃⁻ |
H₂SO₄ | HSO₄⁻ |
HF | F⁻ |
H₂CO₃ | HCO₃⁻ |
H₂O (acts as acid) | OH⁻ |
Base | Conjugate Acid |
|---|---|
H₂O (acts as base) | H₃O⁺ |
NH₃ | NH₄⁺ |
CO₃²⁻ | HCO₃⁻ |
OH⁻ | H₂O |
F⁻ | HF |
💡 Helpful Tips
If it loses H⁺, it’s an acid; the leftover is its conjugate base.
If it gains H⁺, it’s a base; the result is its conjugate acid.
The acid and conjugate base always differ by 1 H⁺.
The base and conjugate acid also differ by 1 H⁺.
Only strong acid produced in human body = hydrochloric acid HCl