Unit 7 P2 Skills of Chemistry >
Review part 1 here
7.4 and 7.5 Equations Introduction/Types of Reactions
Chemical equations represent chemical reactions, and it made up of their reactants, products, and the conditions in which the reaction occurs.
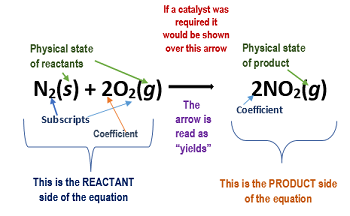
Reactants- Always written on the left side of the equation, these symbols are able to represent atoms, elements, or compounds. In this example, nitrogen and oxygen are the reactants.
Products- The products of an equation are the new substances created from the chemical reaction. The reactants are rearranged to do this and are written on the right of a chemical equation. In this example, Nitrogen dioxide is the product.
Physical state-* The physical state of reactants and products can be seen in parentheses, besides its respective element/compound/atom.
Some basic symbols within them can be identified as: Solid (s), Liquid (l), Gas (g)
Coefficients- Coefficients can be seen on the left side of an element or compound, representing how the number of substances needed for the chemical reaction to occur (does not need to be written if equal to 1). These symbols are also majorly used to balance equations.
Arrow- There are various kinds of arrows, but the basic one is the right arrow: an arrow from the reactants to products, indicating that the reactants are becoming products.
Catalyst- A catalyst quickens a chemical reactant without being used up.
Subscript- Subscripts are written on the bottom right side of an atom. In the example above with 2NO₂, the coefficient 2 belongs to the whole compound, but the subscript 2 only belongs to oxygen. So in total, there would be 2 nitrogen atoms and 4 oxygen atoms (because the subscript and coefficient were multiplied).
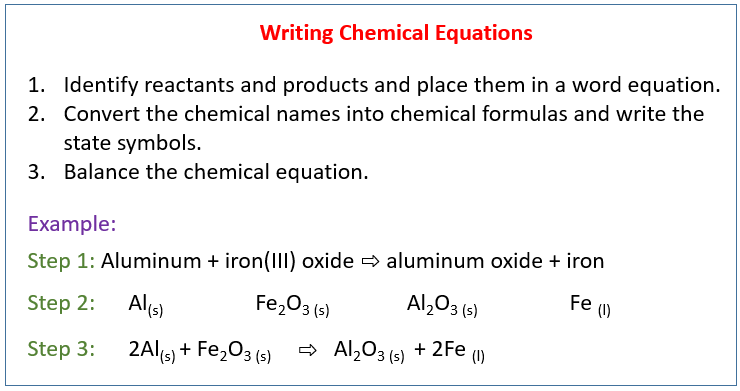
Writing Chemical Equations
To write a chemical equation, follow these steps:
- Identify the reactants and products of the reaction.
- Write the chemical formulas of the reactants on the left-hand side of the equation.
- Write the chemical formulas of the products on the right-hand side of the equation.
- Balance the equation by adjusting the coefficients of the reactants and products to ensure that the number of atoms of each element is the same on both sides of the equation.
For example, the reaction between hydrogen gas and oxygen gas to form water can be represented by the following equation:
2H2(g) + O2(g) → 2H2O(l)
This equation shows that two molecules of hydrogen gas (H2) react with one molecule of oxygen gas (O2) to form two molecules of water (H2O).
Types of Reactions
There are several types of chemical equations, including:
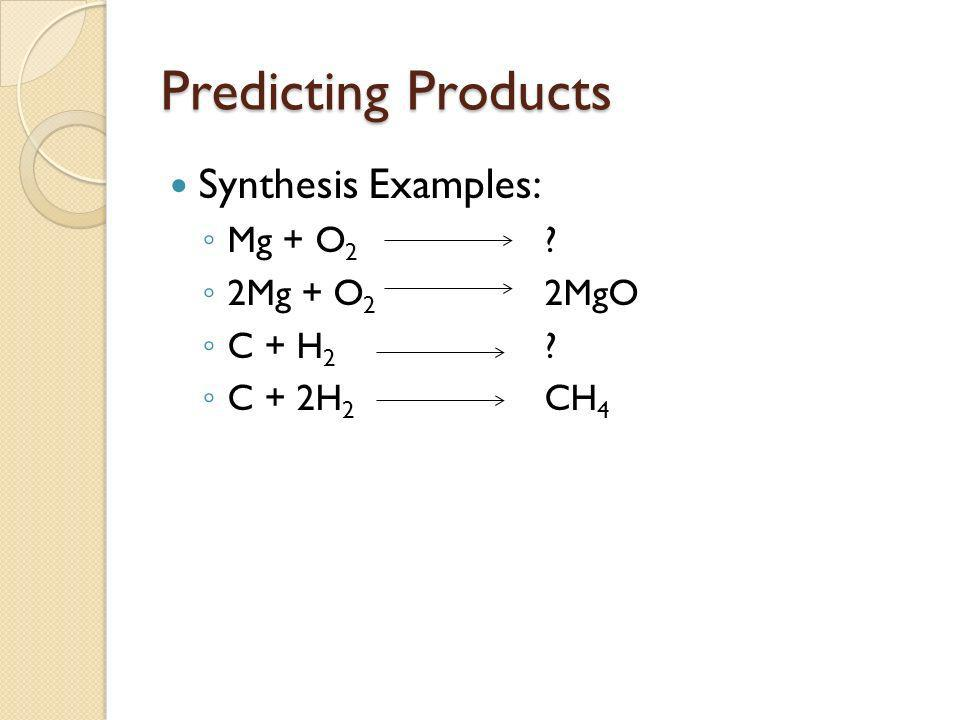
Synthesis reactions: A + B → AB
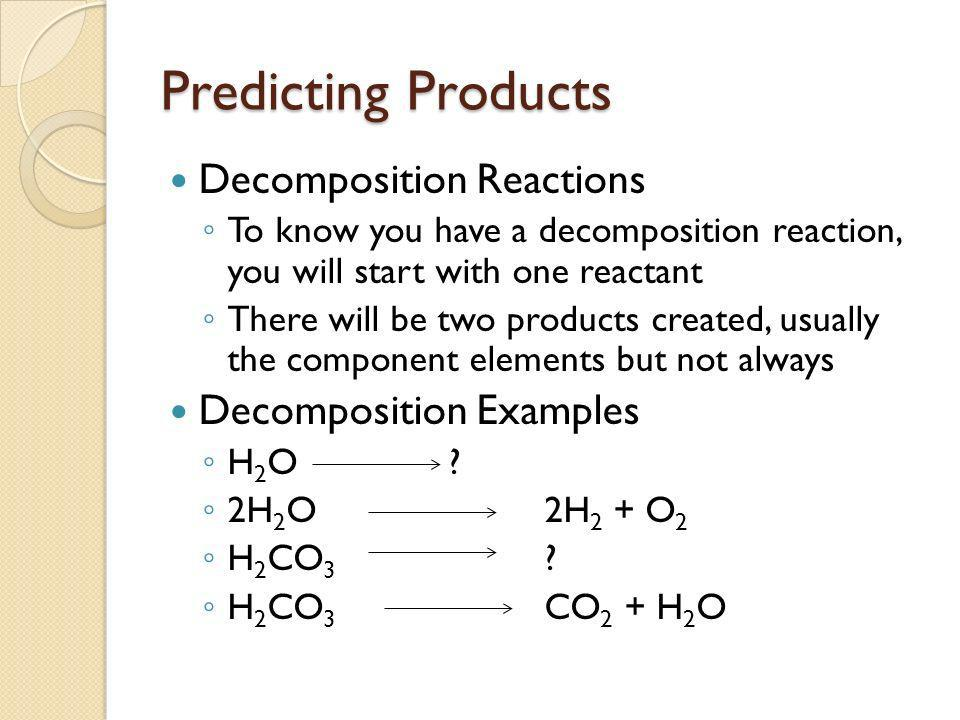
Decomposition reactions: AB → A + B
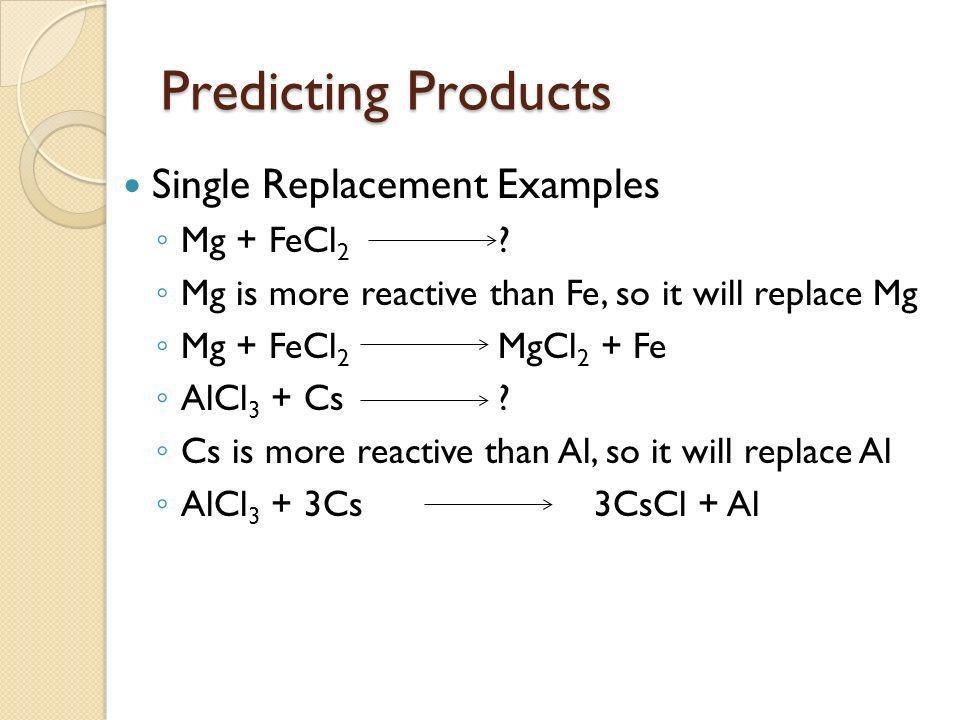
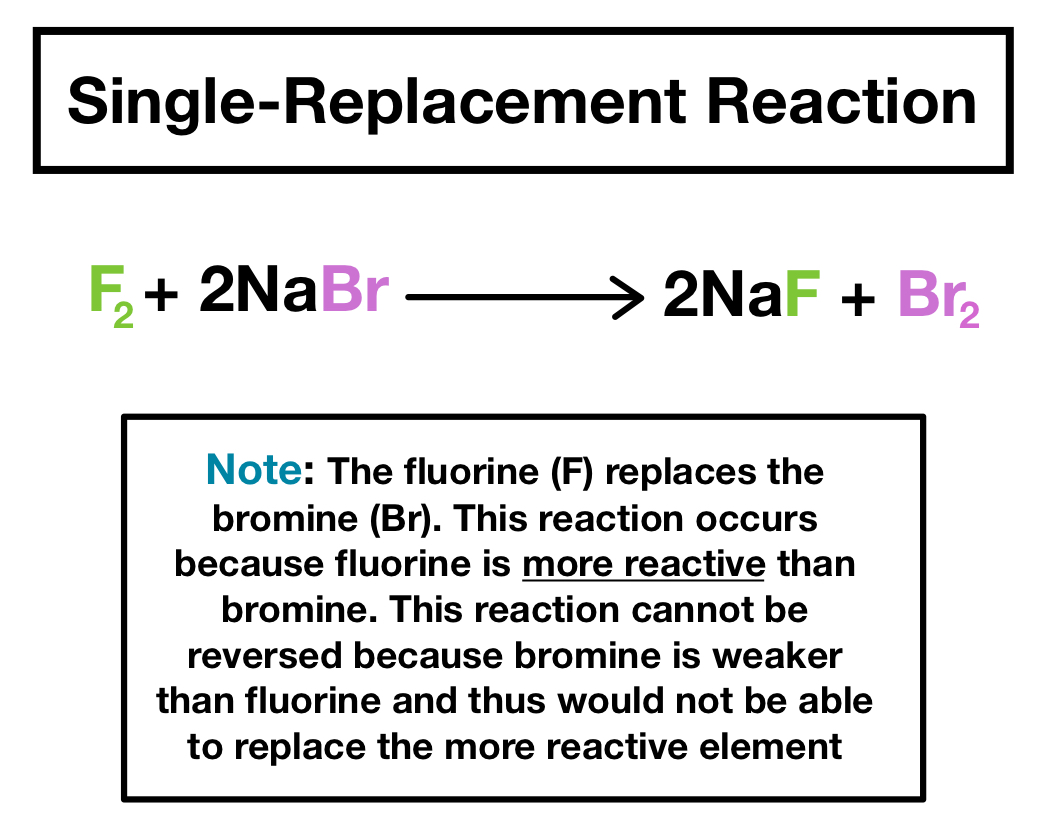
Single displacement reactions: A + BC → AC + B
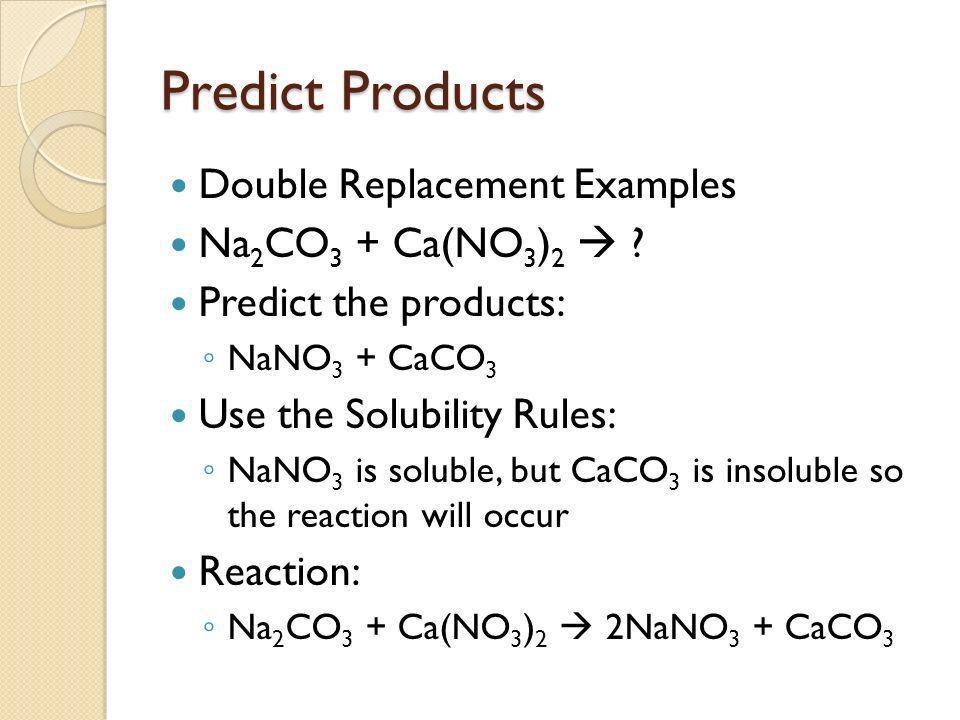
Double displacement reactions: AB + CD → AD + CB
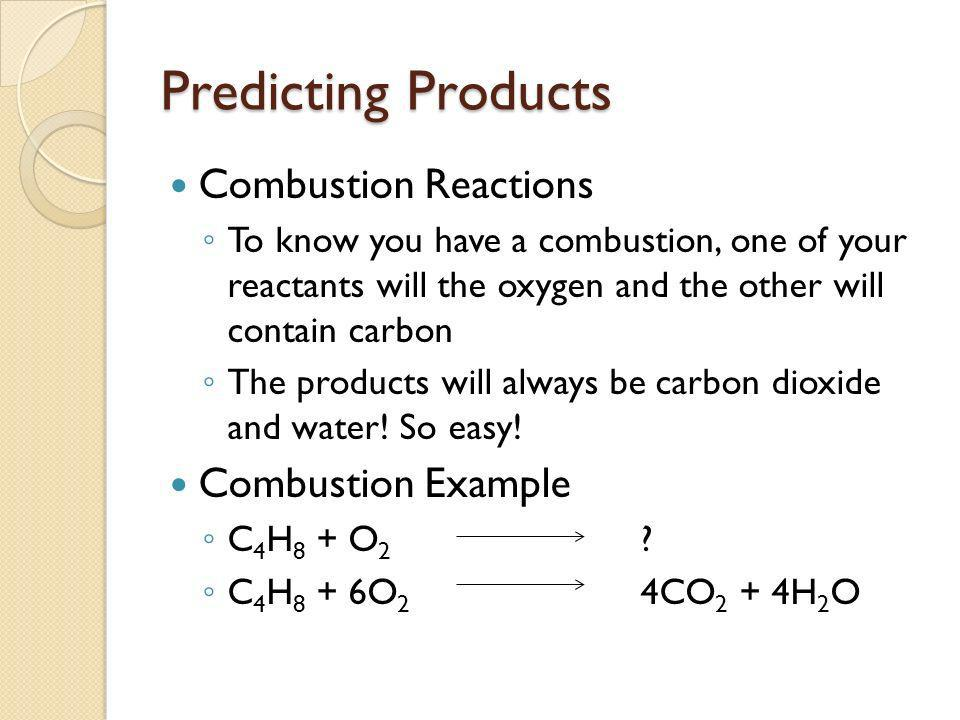
Combustion reactions: CxHy + O2 → CO2 + H2O

| Types | How to Recognize | Factors | Likely Products |
|---|---|---|---|
| Synthesis Combination | Two simple substances combine to form a more complex substance | Multiple (2 substances) → One (complex substance) | A more complex compound |
| Decomposition Analysis | A more complex reactant breaks down into simpler substances | One (complex substance) → Multiple (2 substances) | Simpler products |
| Single Replacement | A single uncombined element replaces another element in a compound | Two reactants → two products | Element replaces another element in a compound |
| Double Replacement | Parts of two ionic compounds switch places to form a new compound | Two compounds → Two compounds | Two anions swap places in a compound |
| Combustion | A form of synthesis where an exothermic reaction occurs, typically resulting and is indicated by soot (carbon) or CO and H2O formation | Similar to synthesis, but involves O2, and the release of H2O and CO2 | CO2, H2O |
Each type of reaction has its own unique characteristics and can be identified by the reactants and products involved.
Practice identification here (highly recommended)
How do we know if a chemical reaction is possible?
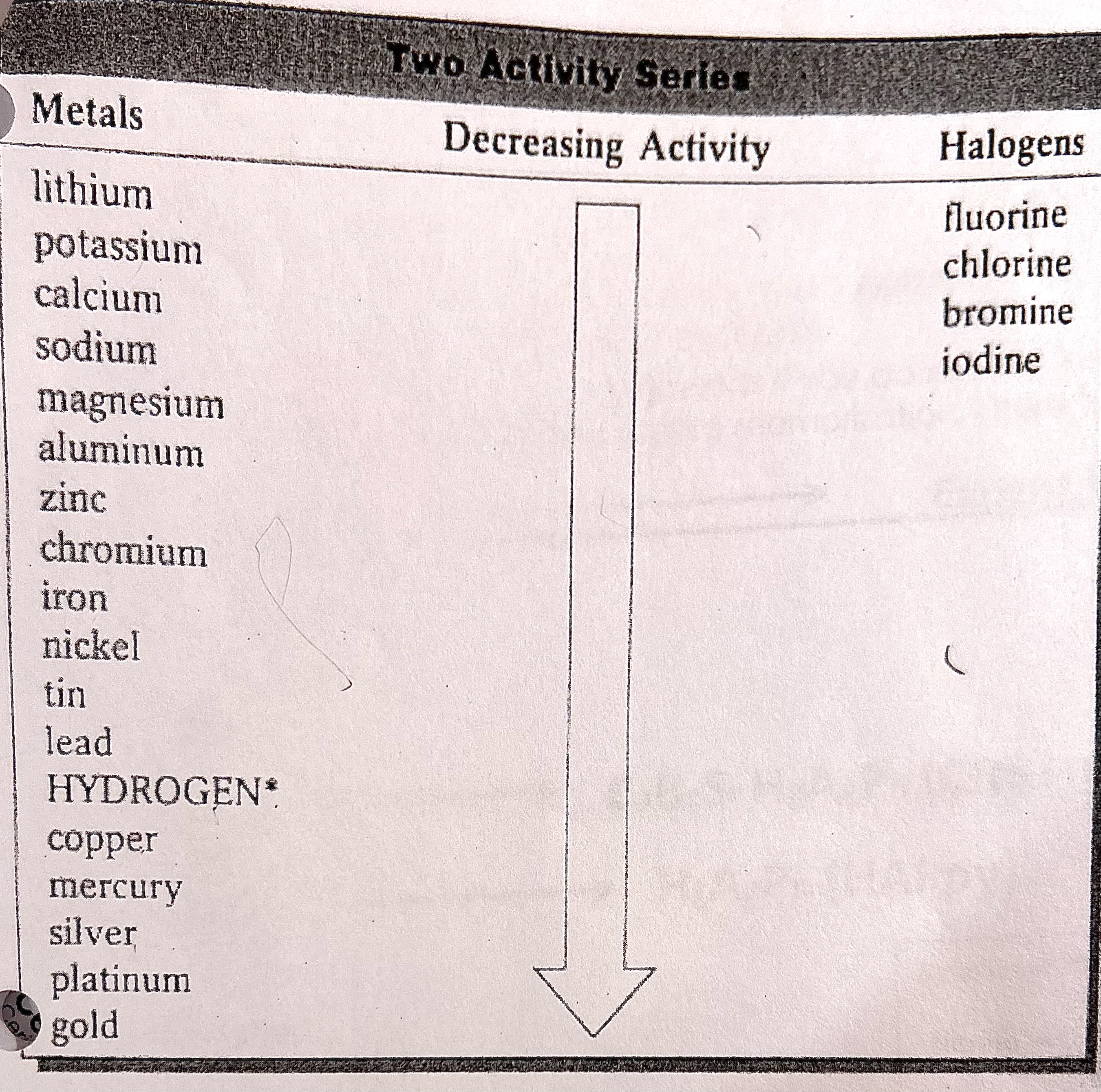
To see if a chemical equation is possible, you first need to determine if the substance replacing the other substance has a higher reactivity level. Only then will the reaction be possible. In other words, If the metal or nonmetal in the reactant is higher on the activity series than the metal or nonmetal in the product, the reaction is possible.
You can use a reference sheet to determine which element has the higher reactivity, such as this one (memorization):
Watch this video if you need more help
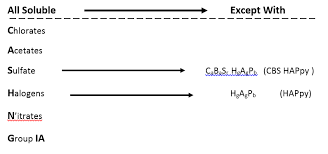
*Solubility rules:
Solubility rules predict if a compound will dissolve in a solvent and determine the state of matter of a compound as some are only soluble in certain solvents or conditions. If a compound is insoluble in water but soluble in ethanol, it will likely be a solid at room temperature.
Rules:
Alkali metal (NA+, K+), NH4+ are all soluble
NO3- Salts are all soluble
Cl-, Br-, I- salts are all soluble (except Ag+, Pb2+, and Nh2+2
SO2-4: BaSO4, CaSO3, PbSO4, HgSO4, are insoluble, others are soluble
OH-, Ca(OH)2, Ba(OH)2 are lightly soluble, except alkali metal salt, all others are insoluble
SO2-3, S2-, CO2-3, PO3-4, usually all insoluble except alkali metal/ammonium salt

Diatomic molecules are mostly gases, hydrogen, oxygen, fluorine, nitrogen, and chlorine. But the last two elements, bromine is liquid and iodine is liquid. (HOFBrINCl)
Every other element is either solid or liquid.
All metals except for Mercury (liquid) are solid
Diatomic molecules are all gases except for bromine (liquid) and iodine (solid).
Diatomic Molecules?
Diatomic molecules are molecules composed of two atoms of the same element. The seven diatomic molecules are H2, N2, O2, F2, Cl2, Br2, and I2 (HOFBrINCl). When using diatomic molecules in chemical equations, it is important to remember that they exist as a pair and cannot be broken apart. For example, when writing the formula for hydrogen gas, it should be written as H2, not just H. When balancing chemical equations, diatomic molecules should be treated as a single unit.
7.6 Word to balanced
To have a balanced equation, each element on both sides of the arrow must have the same amount. For example in 2H2(g) + O2(g) → 2H2O(l), the hydrogens and oxygen must have the same number on both sides. This is more of a guess-and-check process, so just practice it.
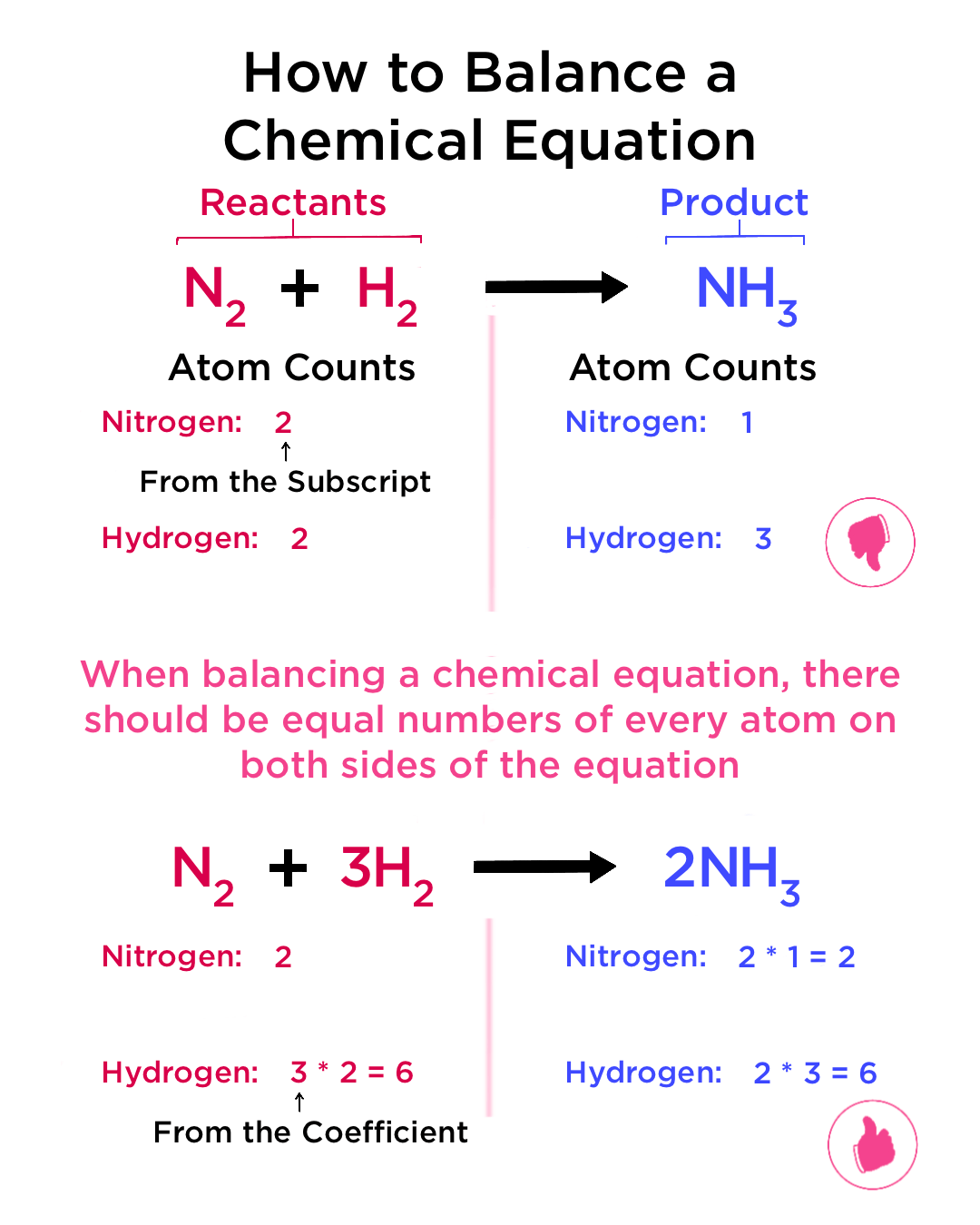
For word problems:
Practice balancing here (highly recommended)
7.7 Predicting Products
Decide what type of reaction it would be. Write down its name.
Based on the reaction type, decide what the product would be.
Be careful to get the product formulas correct. Remember: writing formulas for ionic
compounds, how to write elements (see chart given), and the charge an element will get (positive
or negative).Balance the Chemical Equation.
Refer back to “Types of Reactions” above to help with finding the product.
For multiple-choice practice use this
For open-ended practice, use this and click of the “predicting products” checkbox
Examples: