Module #2
Eukaryotic Features
A network of internal membranes → the endomembrane system
Includes
Nuclear envelope
defines boundary of the nucleus and contains an inner and outer membrane with nuclear pores
Proteins
Functions of proteins
At as enzymes →
Aid in support
Support → cytoskeleton within the cell or proteins
Defense -) antibodies, complement proteins
Amino Acid Structure
Proteins consist of amino acids that are covalently linked into linear polymers → a.k.a. polypeptides (protein = polypeptide)
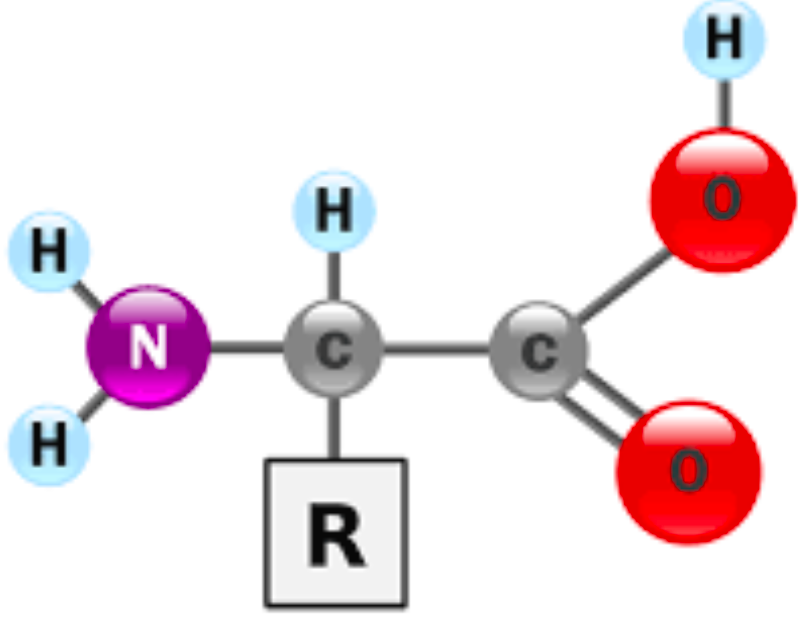
central (alpha - a) carbon is covalently linked to four groups
Carboxyl (-COOH)
Amino (-NH2)
Hydrogen (H)
R group (side chain)
Each amino acid has a unique R group
Joining Amino Acids
At physiological pH (7.4) the carboxly group and amino groups are ionized → amino acids have both positive and negative charges
A zwitterion
Amino acid are joined by a covalent bond
peptide bond
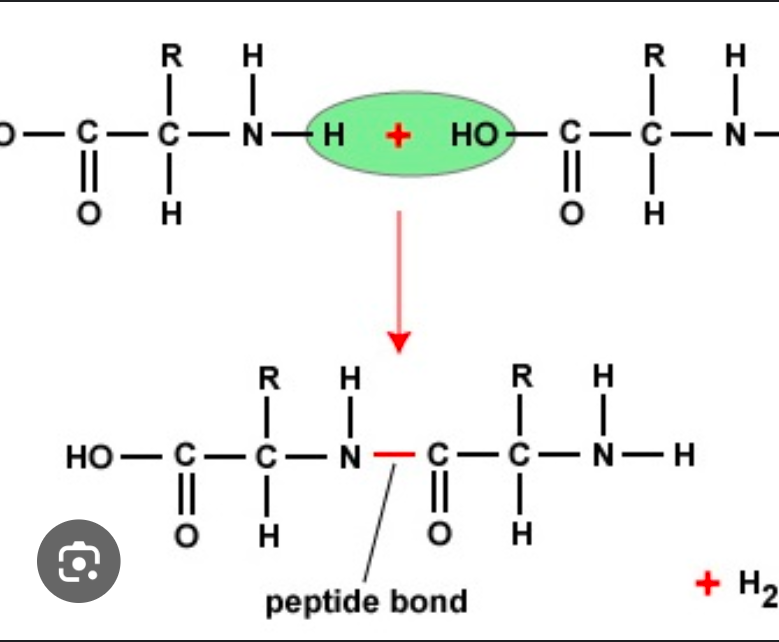
When peptide bonds, form the carboxyl group releases an oxygen atom, and the nitrogen loses loses two hydrogen atoms to produce a water molecule
20 genetically encoded amino acid monomers (see module 5) → the order provides information carried out by the protein
Sometimes two or more polypeptide chains must combine to form a mature protein
Nucleic Acids
General Info
Nucleic Acids are information molecules → encode genetic info in the sequence of nucleotides
Two types of nucleic acids
Deoxyribonucleic acids → DNA
The genetic material in all cellular organisms
Contains information used to direct protein synthesis
Ribonucleic acid → RNA
Multiple functions → key player in protein synthesis & regulation of gene expression
Nucleotides
DNA and RNA are polymers of nucleotides, which are composed of:
A nitrogen containing base that is one of two types
Pyrimidine base that contains a single ring → cytosine (C), thymine (T), and uracil (U)
prymidines CUT
Purine base that contains a double ring → adenine (A) and guanine (G)
Pure As Gold
A 5-carbon (pentose) sugar
In DNA → deoxyribose
in RNA → ribose
One of more phosphate group
Nitrogenous Bases
DNA uses the bases
A, T, C, G
RNA uses the bases
A, U, C, G
Order of the nitrogenous bases determines the information carried in DNA & RNA molecules
Structure
A nucleoside has just a base and a sugar
Functions
Are the monomers of DNA & RNA
Important signal molecules within cells → example cyclic adenosine monophospate (cyclic AMP or cAMP)
Transfer of energy in metabolism → cleave off terminal phosphate group to release stored energy
Act
Connection Nucleotides
Covalent linkage betweenthe phosphate group of one nucleotide to the sugar unit 3’-OH on another → forms a phosphodiester bond
Formation releases a water molecule
Formation of the bond also establishes directionality/polarity of the strand
Beginning of the chain → 5’ end
New nucleotides can be added to the → 3’ end
Always 5’→ 3’ (IMPORTANT)
DNA
DNA consists of two strands of nucleotides twistde around each other to form a double helix
Sugar-phosphate backbone wraps around the outside → antiparallel arrangement
Complementary bases face inwards with H-bonds forming between bases
Complementary Base Pairing:
A-T
C-G
Purine-Pyrimidine
It is beneficial for one strand to create H-Bonds with their complementary strand because it allows DNA to “unzip” down the middle
Hydrogen bonds are weak bonds (individually)
Carbohydrates
Carbohydrates (sugars) are made up of C, H, O atoms
Usally in a ration of 1:2:1 (CH2O)
Serve as a major source of energy for metabolism, but also serve as structural molecules → e.g. cellulose in plants
Most come in 5 or 6 carbon sugars
6-Carbon Sugars
All 6-carbon sugars have the same chemical formula: C6H12O6
Differ in configuration → are isomers so they are funtionally differnt from one another
Simple sugars → monosaccharides
mono = 1
Cyclic Monosaccharides
In cells, virutally all monosaccaharides are in cyclic form
The cyclic structure forms when one of the linear molecule binds to another
Linking Sugars
The covalent linkage between monosaccharides → glycosidic bond
Formation of these bonds results in the release of a water molecule
Forms between C1 of one monosaccharide and -OH group on the carbon of a different monosaccharide
Covalently linking two monosaccharides → forms a disaccharide (di = “two”)
e.g. sucrose (C12H22O11)
A few monosaccharides joining → forms oligosaccharides (oligo = “dew”)
Can be attached to:
Proteins forming → glycoproteins
Lipids forming → glycolipids
More than two monosaccharide monomers
Lipids
Lipids are a chemically diverse group of molecule → it is the only macromolecule that is not a polymer
They are all grouped together because they share the same physical property → hydrophobic
Cells use different lipids in the following ways:
Triacylglycerol
Major component of animal fat and vegetabe oil
Made up ofL
Three fatty acids → a type of lipid made up of a long chain of carbons attached to a carboxyl group (-COOH) at one end
Has carbon-carbon double bonds = unsaturated
Steroids
Steroids are composed of many carbon atoms bonded to characteristic four fused rings
Hydrophobic
Cholesterol is a component of animal cell membranes
Phospholipids
A major component of cell membranes
Made up of:
Glycerol backbone attached to a polar phosphate group → hydrophilic
Two fatty acid tails which are nonpolar → hydrophobic
Molecules with both hydrophilic& hydrophobic regions are called amphipathic (KEY)