Strong Acids and Bases
Identifying Acids and Bases
Acids: Typically have hydrogen in front of their chemical formula.
Examples: HCl (hydrochloric acid), HF (hydrofluoric acid), HC2H3O2 (acetic acid).
Bases: Typically have a hydroxide ion (OH^-).
Examples: NaOH (sodium hydroxide), KOH (potassium hydroxide).
Exceptions:
Hydrogen next to a metal (e.g., NaH - sodium hydride) is a base.
Hydrogen attached to a nonmetal is typically an acid.
Charge:
Positive hydrogen indicates an acid.
Negative hydrogen indicates a base.
Acids tend to be positively charged, bases negatively charged.
Arrhenius Definition
Acids: Substances that release hydrogen ions (H^+) into solution.
Hydrogen ions in water are equivalent to hydronium ions (H_3O^ +) because H^+ doesn't exist by itself in water; it bonds to water.
Bases: Substances that release hydroxide ions (OH^-) into solution.
Bronsted-Lowry Definition
Acids: Proton donors.
Bases: Proton acceptors.
Example 1: Hydrochloric Acid in Water
Reaction: HCl + H2O \rightarrow Cl^- + H3O^ +
HCl is the Bronsted-Lowry acid (proton donor).
H_2O is the Bronsted-Lowry base (proton acceptor).
Cl^- is the conjugate base (formed after HCl donates a proton).
H3O^ + is the conjugate acid (formed after H2O accepts a proton).
Example 2: Ammonia in Water
Reaction: NH3 + H2O \rightarrow NH_4^ + + OH^-
NH3 is the base (proton acceptor, turns into NH4^ +).
H_2O is the acid (proton donor, turns into OH^-).
NH_4^ + is the conjugate acid.
OH^- is the conjugate base.
Writing Conjugate Acids and Bases
Conjugate Acid: Add H^+ and increase the charge by 1.
Example: Water (H2O) becomes hydronium (H3O^ +).
Conjugate Base: Remove H^+ and decrease the charge by 1.
Example: Water (H_2O) becomes hydroxide (OH^-).
Examples:
Ammonia (NH_3):
Conjugate acid: NH_4^ +
Conjugate base: NH_2^-
Dihydrogen Phosphate (H2PO4^-):
Conjugate acid: H3PO4
Conjugate base: HPO_4^{2-}
pH Scale
Typically ranges from 0 to 14, but can go beyond these numbers.
pH = 7: Neutral solution.
pH < 7: Acidic solution (e.g., pH = -2 is very acidic).
pH > 7: Basic solution.
pH Calculations
pH = -log[H_3O^+] (negative log of hydronium ion concentration).
pOH = -log[OH^-] (negative log of hydroxide ion concentration).
pH + pOH = 14 (at 25 degrees Celsius).
[H_3O^+] = 10^{-pH} (hydronium ion concentration).
[OH^-] = 10^{-pOH} (hydroxide ion concentration).
Strong vs Weak Acids
Strong Acids: Ionize completely in solution.
Form strong electrolytes (conduct electricity well).
Ionize almost 100%.
Weak Acids: Partially ionize in solution (less than 5%).
Form weak electrolytes.
Common Strong Acids
HCl (hydrochloric acid)
HBr (hydrobromic acid)
HI (hydroiodic acid)
HNO_3 (nitric acid)
H2SO4 (sulfuric acid)
HClO_4 (perchloric acid)
Note: HF (hydrofluoric acid) is a weak acid, not a strong acid.
Weak Acids
NH_4^ + (ammonium ion)
HC2H3O_2 (acetic acid)
Cyanic acid
Nitrous acid
Sulfurous acid
Oxyacids
For oxyacids, the acid with more oxygen atoms is more acidic.
Sulfuric acid (H2SO4) is stronger than sulfurous acid (H2SO3).
Nitric acid (HNO3) is stronger than nitrous acid (HNO2).
Perchloric acid (HClO4) > chloric acid (HClO3) > chlorous acid (HClO_2) > hypochlorous acid (HClO).
This trend does not apply to acids without oxygen (e.g., HCl).
Chemical Reactions with Strong and Weak Acids
Strong Acids: Use a single arrow to show complete ionization.
Example: HCl + H2O \rightarrow Cl^- + H3O^ +
Weak Acids: Use a double arrow to show partial ionization and equilibrium.
Example: HF + H2O \rightleftharpoons F^- + H3O^ +
Strong vs Weak Bases
Strong Bases: Soluble ionic compounds that ionize completely.
Examples: KOH (potassium hydroxide), NaOH (sodium hydroxide), Ba(OH)_2 (barium hydroxide).
Form strong electrolytes.
Weak Bases: Insoluble compounds that ionize partially (less than 1%).
Example: Al(OH)_3 (aluminum hydroxide).
Ammonia (NH3) and conjugate bases of weak acids (e.g., fluoride, nitrite, acetate, HSO3^-).
Other Strong Bases
Oxide (O^{2-}): A stronger base than hydroxide.
O^{2-} + H_2O \rightarrow 2OH^-
Hydride (H^-): A strong base that produces hydrogen gas and hydroxide ions when reacting with water.
H^- + H2O \rightarrow H2 + OH^-
Base Strength Comparison
O^{2-} > OH^- > H2O > H3O^+
Oxide is the most basic, hydronium is the most acidic.
Hydride is a stronger base than hydroxide.
Reaction Mechanisms
Oxide and Water: Oxide uses a lone pair to take a hydrogen from water, forming two hydroxide ions.
Hydride and Water: Hydride (with a negative charge) is attracted to the partially positive hydrogen in water, forming hydrogen gas and hydroxide ions.
Properties of Acids and Bases
Taste:
Acids taste sour (e.g., lemons).
Bases taste bitter.
Feel:
Bases feel slippery.
Indicators:
Acids turn blue litmus paper red.
Bases turn red litmus paper blue.
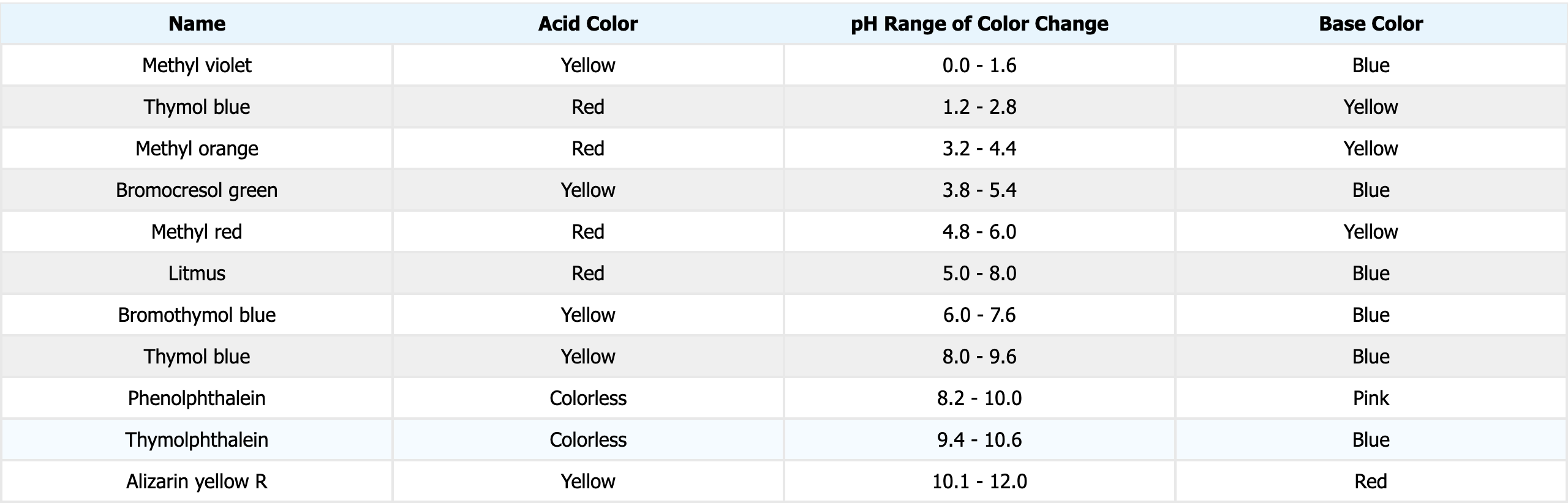
pH of Solutions
Acidic: pH < 7
Neutral: pH = 7
Basic: pH > 7
Electrical Conductivity
Strong acids and strong bases are strong electrolytes; they conduct electricity very well due to complete ionization.
Weak acids and weak bases are weak electrolytes; they conduct a small amount of electricity due to partial ionization.
Electrical conductivity of a strong acid solution > electrical conductivity of a weak acid solution.
Reactions with Active Metals
Acids react with active metals to produce hydrogen gas.
Example: Zn + 2HCl \rightarrow ZnCl2 + H2
Active metals: Zinc, aluminum, iron, nickel, sodium (but sodium is too reactive with water).
Inactive metals (copper, silver, gold) do not react with acids to produce hydrogen gas.
Definitions of Acids and Bases (Recap)
Arrhenius:
Acids release H^+ ions in solution.
Bases release OH^- ions in solution.
Bronsted-Lowry:
Acids are proton donors.
Bases are proton acceptors.
Lewis:
Acids are electron pair acceptors.
Bases are electron pair donors.
Acid Dissociation Constant (Ka)
Example: Hydrofluoric Acid in Water
Reaction: HF(aq) + H2O(l) \rightleftharpoons H3O^+(aq) + F^-(aq)
Ka is the acid dissociation constant.
Ka = \frac{[H_3O^+][F^-]}{[HF]} (products over reactants).
Liquids and solids are not included in the equilibrium expression.
As Ka increases, the strength of the acid increases.
As Ka increases, the pKa value decreases.
Strong acids have large Ka values, small pKa values.
pKa = -log(Ka)
Base Dissociation Constant (Kb)
Example: Ammonia in Water
Reaction: NH3(aq) + H2O(l) \rightleftharpoons NH_4^+(aq) + OH^-(aq)
Kb is the base dissociation constant.
Kb = \frac{[NH4^+][OH^-]}{[NH3]}
pKb = -log(Kb)
Amphoteric Substances
Substances that can act as both an acid and a base.
Example: Water (H_2O)
Example: Dihydrogen Phosphate (H2PO4^-)
As a base: H2PO4^- + HF \rightarrow H3PO4 + F^-
As an acid: H2PO4^- + NH3 \rightarrow NH4^+ + HPO_4^{2-}
When an acid loses a hydrogen, it forms the conjugate base.
When a base gains a hydrogen, it forms the conjugate acid.
Autoionization of Water
Water reacts with itself.
Reaction: 2H2O(l) \rightleftharpoons H3O^+(aq) + OH^-(aq)
Kw = [H_3O^+][OH^-] (autoionization constant for water).
Kw is temperature-dependent (increases with temperature).
At 25 degrees Celsius, Kw = 1 \times 10^{-14}.
[H_3O^+][OH^-] = 1 \times 10^{-14}
pH + pOH = 14
pKa + pKb = 14
Ka * Kb = Kw = 1 \times 10^{-14}
Practice Problems
Problem 1
The H_3O^+ concentration in a solution is 4 \times 10^{-3}. What is the pH of the solution?
pH = -log[H_3O^+]
pH = -log(4 \times 10^{-3}) = 2.3979 \approx 2.4
Problem 2
The OH^- concentration in a solution is 5.3 \times 10^{-4}. What is the pOH of the solution?
pOH = -log[OH^-]
pOH = -log(5.3 \times 10^{-4}) = 3.2757 \approx 3.28
Problem 3
We're given the hydronium ion concentration, and we want to calculate the hydroxide ion concentration.
[H_3O^+][OH^-] = Kw
[OH^-] = Kw/[H_3O^+]
[OH^-] = (1 \times 10^{-14}) / (2.5 \times 10^{-5}) = 4 \times 10^{-10}
Problem 4
If the Ka of acetic acid (HC2H3O_2) is 1.8 \times 10^{-5}, what is the pKa of the acid?
pKa = -log(Ka)
pKa = -log(1.8 \times 10^{-5}) = 4.745
Problem 5
Which of the following statements is false?
Bases taste bitter and feel slippery. (True)
Acids taste sour and acids react with active metals to produce hydrogen gas. (True)
HCl is a strong electrolyte. (True)
Acids turn red litmus paper blue. (False)
Sodium hydroxide is a strong base, etc. (True)
Problem 6
Which of the following solutions will have the highest pH? (Assuming the same concentration for each).
HBr (strong acid)
HF (weak acid)
NaCl (neutral salt, pH = 7)
NH_3 (weak base)
KOH (strong base)
The higher the pH value, the stronger the base.
Problem 7
The Ka value for HF and acetic acid are 7.2 \times 10^{-4} and 1.8 \times 10^{-5}, respectively. Which acid is stronger: hydrofluoric acid or acetic acid?
As the Ka value increases, the strength of the acid increases. Because 7.2 \times 10^{-4} is larger than 1.8 \times 10^{-5}, hydrofluoric acid is the stronger acid.
Problem 8
Match each term with the correct letter.
Arrhenius acid (releases H+ ions in solution)
Arrhenius base (releases hydroxide ions in solution)
Bronsted-Lowry acid (Proton donor)
Bronsted-Lowry base (Proton acceptor)
Lewis acid (electron pair acceptor)
Lewis base (electron pair donor)