MRTY3119: Week 12 - Stroke and Aneurysms
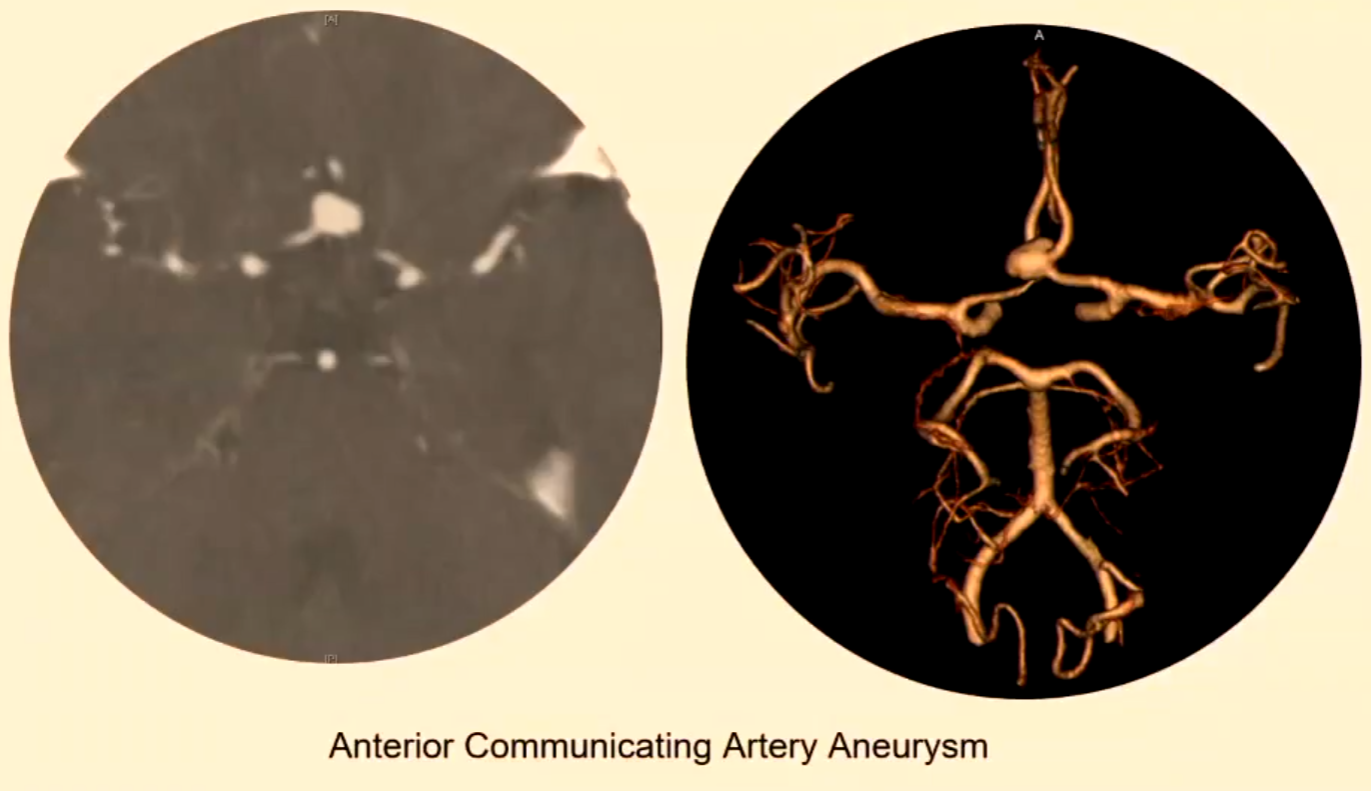
Intracranial Aneurysms
Types and Locations
Saccular: Most common, approximately 90% of intracranial aneurysms.
Commonly involve the Circle of Willis (CoW).
Fusiform: Aneurysms that are characterized by a spindle-shaped dilation of the artery, typically affecting the vertebral and basilar arteries.
Mycotic: Aneurysms resulting from infection, often associated with embolic events or bacterial endocarditis, leading to the weakening of the vessel wall.
Dissecting: Aneurysms that occur when a tear forms in the arterial wall, allowing blood to flow between the layers of the vessel wall, which can result in severe complications such as stroke.
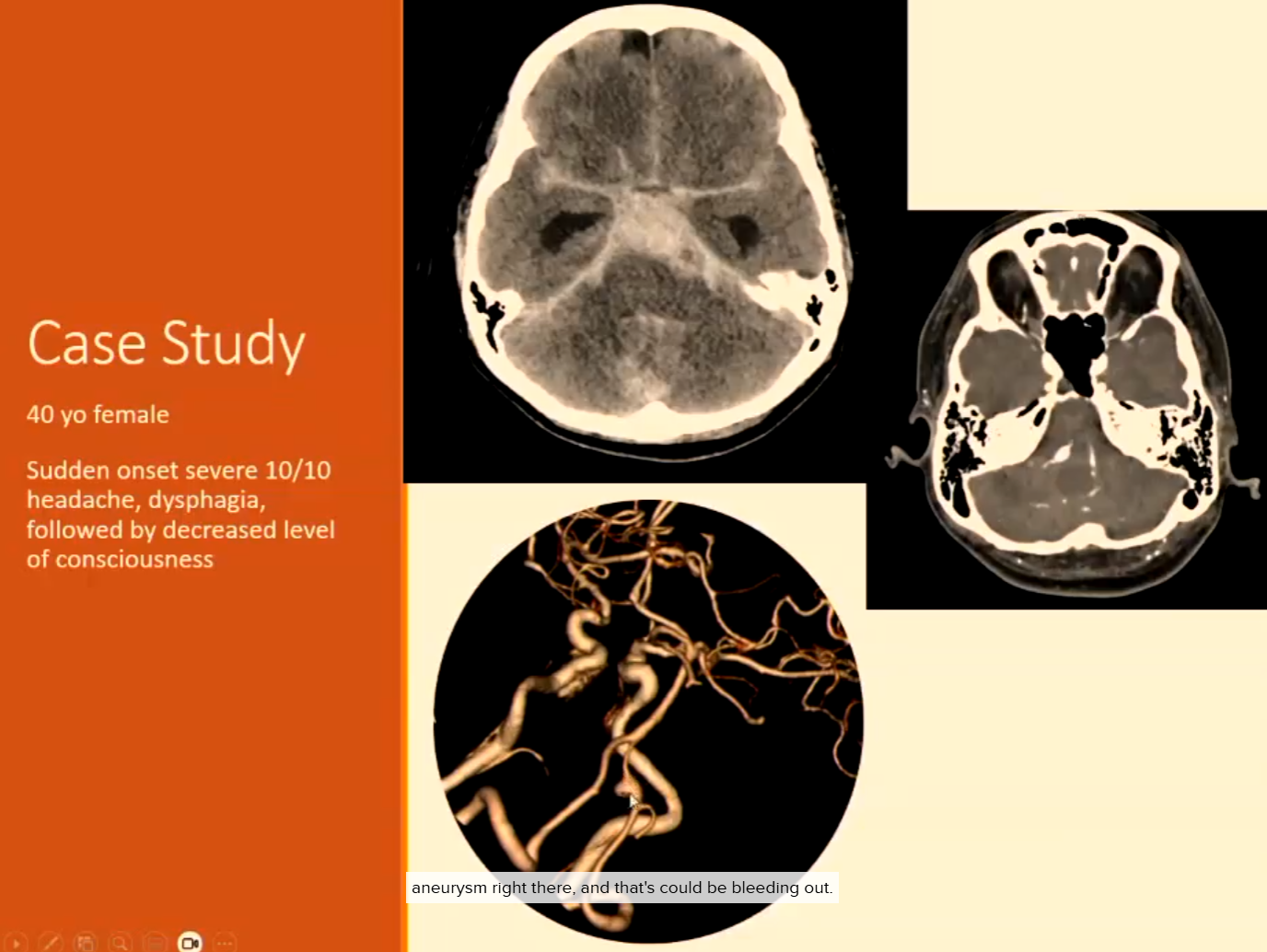
Clinical Signs of Rupture (Subarachnoid Haemorrhage or Intraparenchymal)
Sudden severe “thunderclap” headache
Nausea, vomiting
Neck stiffness
Loss of consciousness
Stroke
Stroke is a generic term for a group of cerebrovascular disorders.
Brain Infarction (Ischaemic Infarct)
Middle Cerebral Artery (MCA) infarct
Lacunar infarct
Watershed infarct (Border infarct)
Infarction: a condition characterized by the obstruction of blood supply to a specific area of the brain, resulting in tissue death.
Haemorrhagic Infarct (Haemorrhagic Stroke or Intracranial Haemorrhage)
Ruptured aneurysm (usually saccular)
Trauma
Vascular malformations
Hypertension
Haemorrhagic infarct: a type of stroke that occurs when a blood vessel in the brain bursts, leading to bleeding in the surrounding tissue and resulting in neuronal damage.
Key Points
Stroke: Sudden onset of symptoms causing various problems.
Common Symptoms: Arm and leg paralysis, speech problems, visual problems, coordination difficulties.
Transient Ischaemic Attacks (TIA): Minor stroke-like attacks with increased risk of future strokes, requiring serious attention.
Stroke Mimics: Conditions that can mimic stroke symptoms and must be taken seriously.
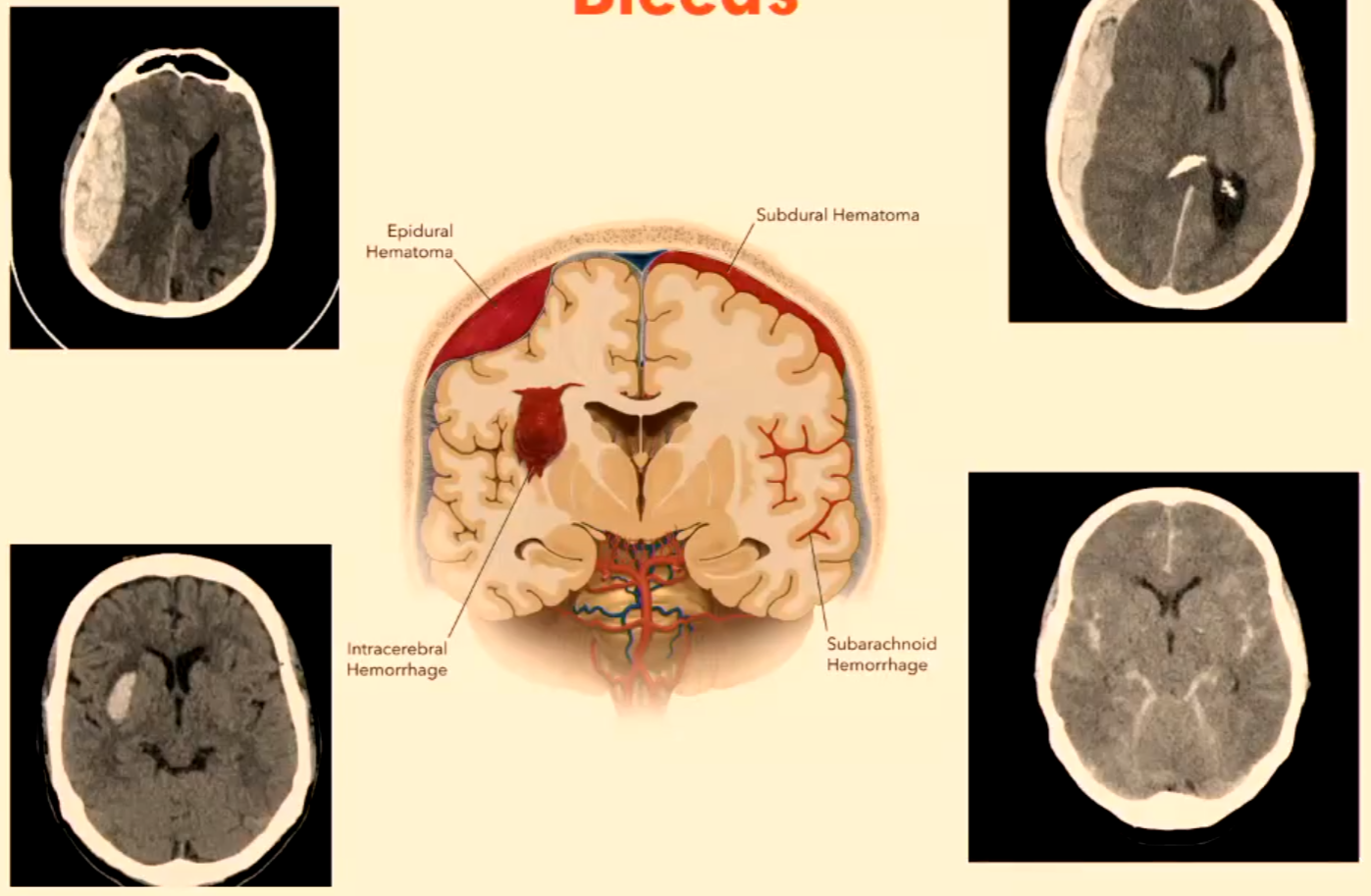
Bleeds
Epidural Hematoma
Subdural Hematoma
Intracerebral Hemorrhage
Subarachnoid Hemorrhage
Acute Stroke Patients
Approximately 15 million strokes per year:
6 million deaths
5 million permanently disabled
4 million recovering
In Australia:
Approximately 2/3 are ischaemic, secondary to:
Large artery atherosclerosis: this is the buildup of fatty deposits in the arteries, which can restrict blood flow to the brain and lead to ischemic strokes.
Cardioembolism: occurs when a blood clot forms in the heart and travels to the brain, blocking a blood vessel and causing a stroke.
Small vessel occlusion: this type of stroke is caused by the blockage of smaller arteries supplying blood to the brain, often due to chronic high blood pressure or diabetes, leading to lacunar infarcts.
1/3 are haemorrhagic:
Intracerebral
Subarachnoid
Stroke Types
Ischaemic Stroke: Artery becomes blocked.
Embolic: Clot forms in the body (commonly the heart), migrates, and blocks an intracranial artery (cardioembolic cause).
Thrombotic: Narrowing of arteries by plaques, significantly impacting blood flow (occlusive atheromatous disease).
Goals of Imaging of Acute Stroke Patient
Differentiate between ischaemic and haemorrhagic stroke.
Determine if brain tissue is viable and suitable for interventional therapy (e.g., thrombolysis or ECR).
Localisation of vascular occlusion.
CT Perfusion
MTT = Mean Transit Time
TTP = Time To Peak
CBF = Cerebral Blood Flow
CBV = Cerebral Blood Volume
Risks of Strokes
Risk Factors
Heredity
High BP (approx. 50%)
Smoking
Diabetes (approx. 15%)
Obesity
Familial hypercholesterolemia
Myocardial infarction
Atrial fibrillation
Congestive heart failure
Alcohol excesses
Oral contraceptives
High anxiety + stress
Causes
Nonvascular (tumours, hypoxia) approx. 5%
Vascular (95%):
Brain infarction
Small vessel disease of penetrating arteries (lacunar infarcts)
Cardiogenic emboli
Haemorrhagic stroke (20%)
Primary SAH (15%)
Vasospasm due to non-traumatic SAH (4%)
Veno-occlusive disease (1%)
Determining Presence or Absence of Stroke
NIHSS (National Institutes of Health Stroke Scale)
13 tests are performed and scored.
Score Interpretation:
0: No Stroke Symptoms
1-4: Minor Stroke
5-15: Moderate Stroke
16-20: Moderate to Severe Stroke
21-42: Severe Stroke
Alberta Stroke Program Early CT Score (ASPECTS).
Application of NIHSS and Imaging
IV tPA: Intravenous injected tissue-type plasminogen activator (thrombolysis).
IA tPA: Intra-arterial injected tPA.
Key Factors: Time, haemorrhage, core, penumbra.
Imaging: CT, CT + MR or MR alone.
NIHSS | % Stroke Population | Treatment | Key Factors | Imaging |
|---|---|---|---|---|
<5 | 55% | None | None | None |
5-10 | 10% | IV tPA | Time, haemorrhage CT | |
>10 | 35% | IV tPA | Time, haemorrhage CT | |
IA tPA | Time, haemorrhage, core, penumbra | CT + MR or MR alone |
Imaging for Stroke and Sensitivity of Modality
Haemorrhage | Major Artery Occlusion | Late Infarct | Early Infarct | Core | Penumbra | |
|---|---|---|---|---|---|---|
CTAngio | + | + | + | - | - | |
CTPerfusion | - | - | - | +/- | + | |
MRI/MRA | + | + | + | + | - | |
MRP | - | - | - | - | - | + |
Alberta Stroke Program (ASPECTS)
10-point scoring system from CT images at ganglionic and supraganglionic sections.
Ganglionic: Involves the basal ganglia region, and points are assigned based on the degree of early ischemic changes observed in this area.
Supraganglionic: This section evaluates the cortical and subcortical areas of the brain, with points awarded depending on the extent of ischemic changes and any associated mass effect.
MCA territory: 1 point for each structure, 1 point deducted for each area with hypodensity.
Score > 6: Better response to reperfusion.
Score < 6: Poorer response.
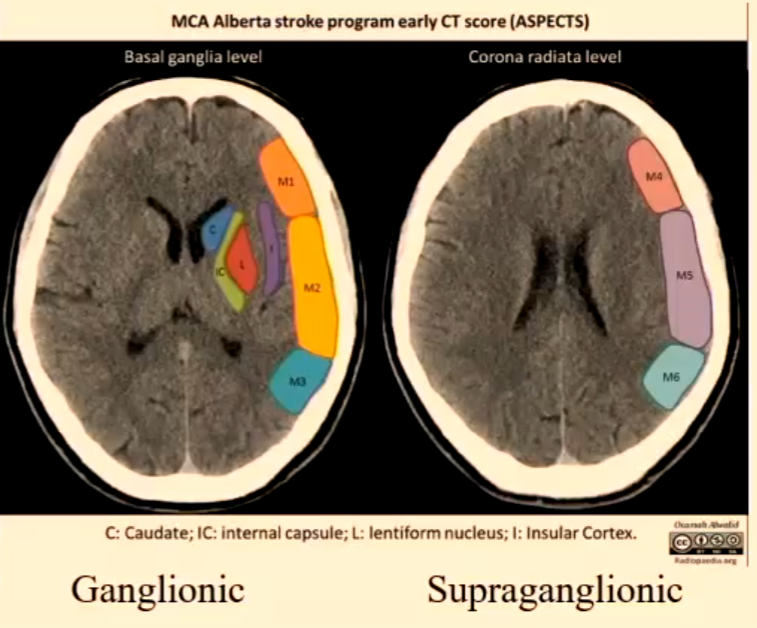
Alberta Stroke Program Early CT Score (ASPECTS) - Terminology
M1: Anterior MCA cortex
M2: MCA cortex lateral to insular ribbon
M3: Posterior MCA cortex
M4: Anterior MCA territory immediately superior to M1
M5: Lateral MCA territory immediately superior to M2
M6: Posterior MCA territory immediately superior to M3
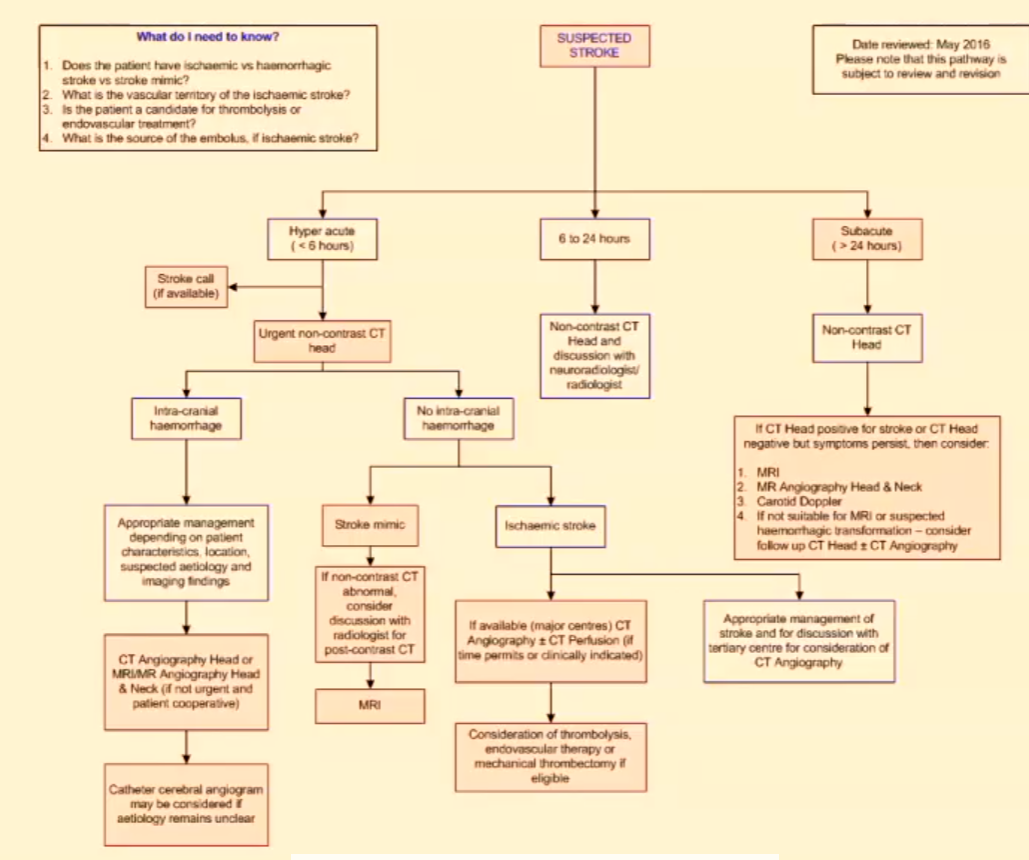
Imaging Pathway for Stroke (Australia)
Does the patient have ischaemic vs haemorrhagic stroke vs stroke mimic?
What is the vascular territory of the ischaemic stroke?
Is the patient a candidate for thrombolysis or endovascular treatment?
What is the source of the embolus, if ischaemic stroke?
(Post Event) Time Classifying of Ischaemic Strokes
HYPERACUTE: 3-6 hours post ictus (relates to time for effective thrombolysis introduction).
Thrombolysis: a treatment that involves dissolving blood clots in order to restore blood flow to the affected area of the brain.
ACUTE: > 6 hours.
SUBACUTE: 3-6 days.
CHRONIC: > 1 week.
Ischaemic Infarct
Temporal lobe – embolis MCA
Acute right middle cerebral artery territorial infarct with oedema and mass effect.
Resultant loss of grey-white matter differentiation.
Hyperdense right MCA (dense MCA sign).
Insular ribbon sign.
Effacement of the right insular.
Disappearing basal ganglia sign.
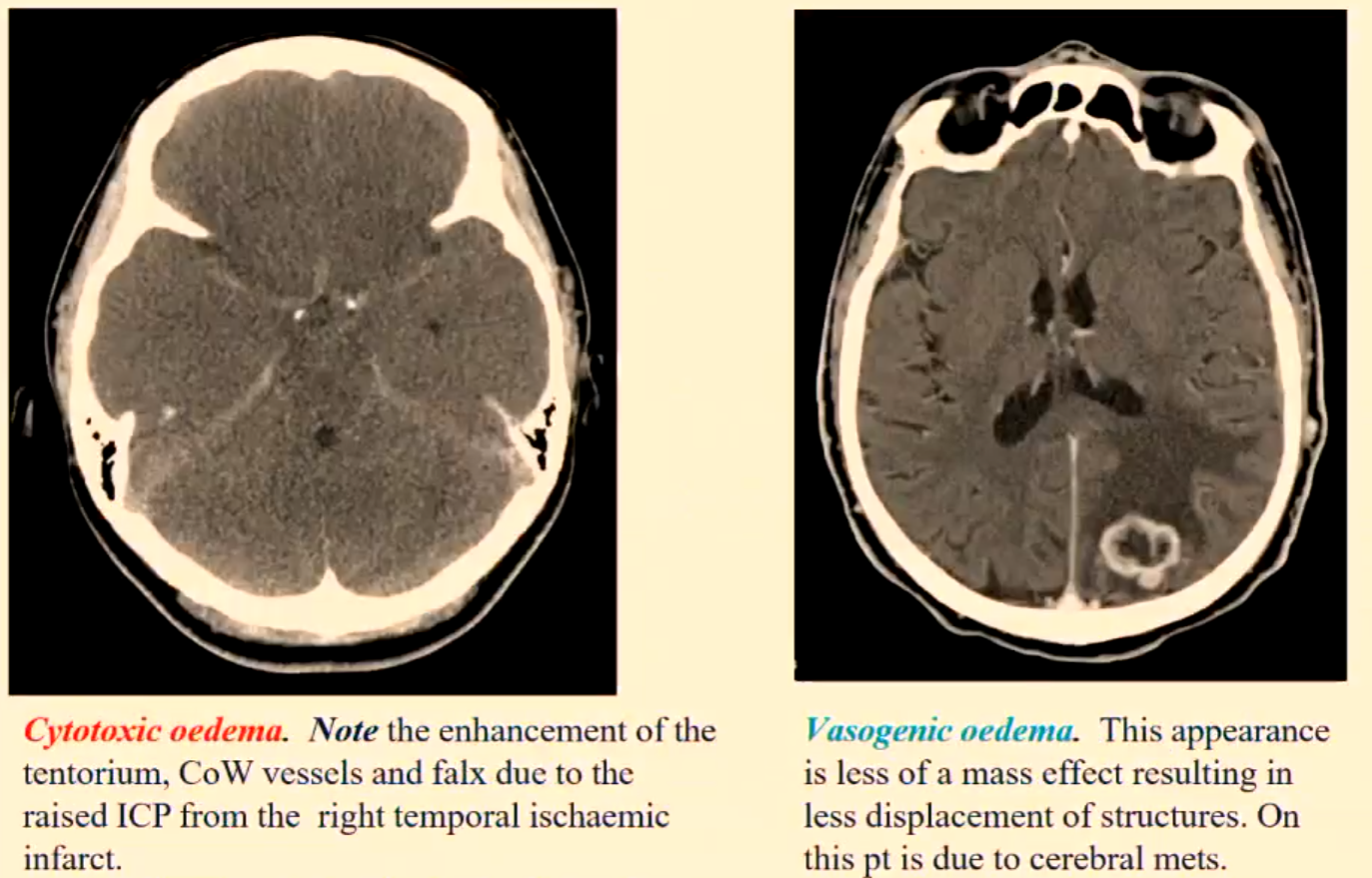
Cerebral Oedema
Cerebral oedema creates a mass effect due to the increase in water and sodium content in the affected area.
This causes a raising of the ICP and is capable of being life threatening.
ICP: Intracranial Pressure, which refers to the pressure within the skull that can influence brain function and result in severe complications if not managed appropriately.
Cerebral oedema is caused by cerebral trauma, massive cerebral infarction, cerebral haemorrhages, brain tumours, brain abscesses, hypoxia, sepsis etc.
CYTOTOXIC OEDEMA – results from the swelling of neurons, glia and endothelial cells, caused by ischaemia and hypoxia.
VASOGENIC OEDEMA – is caused by brain tumours, brain abscesses and infection to a lesser degree trauma and haemorrhage.
Definition of Description Terms in MCA Infarct
Hyperdense MCA sign (HMCAS): Increased attenuation of the proximal portion of the MCA associated with thrombosis of the M1 MCA segment. Early sign of ischaemic stroke (within 90 minutes).
Disappearing basal ganglia sign: Loss of the normal delineation of the basal ganglia.
Insular ribbon sign: Loss of the gray-white interface. The insular is supplied by the insular segment of the MCA and is particularly susceptible to ischaemia.
Cerebral Infarct Territories
MCA
ACA
PCA
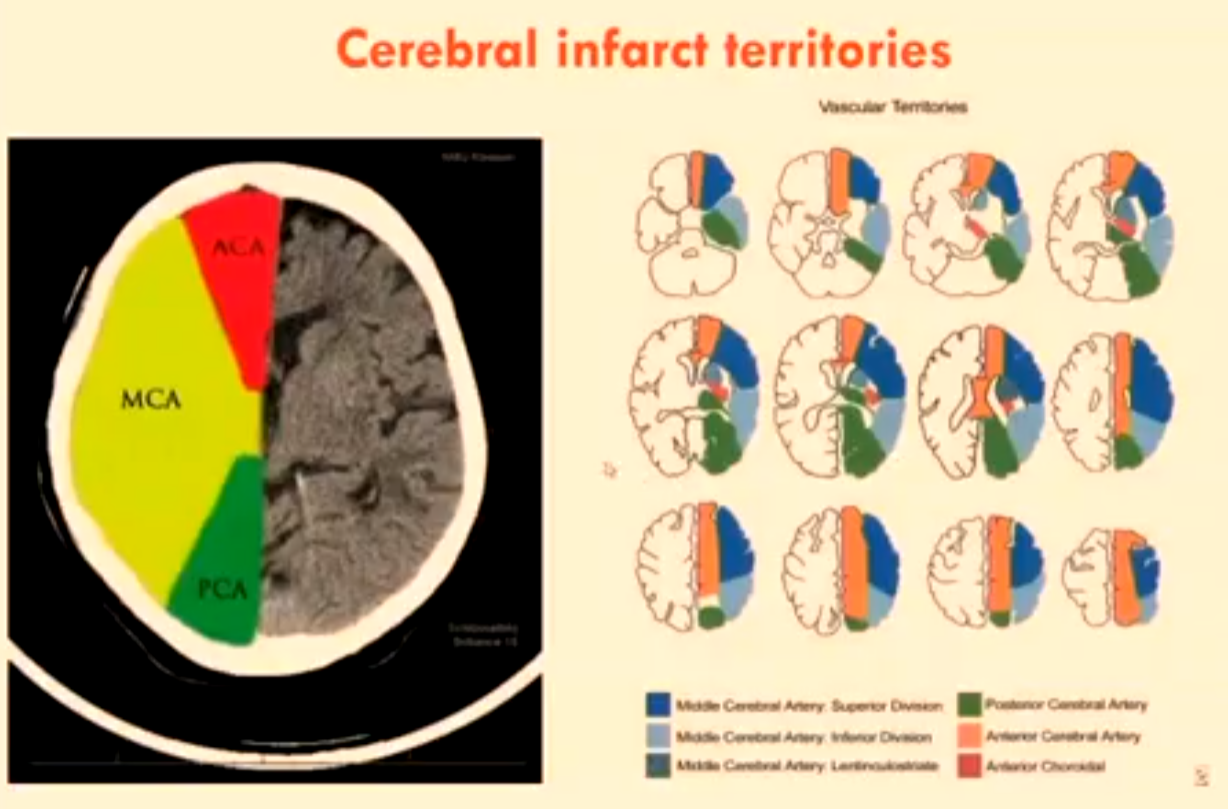
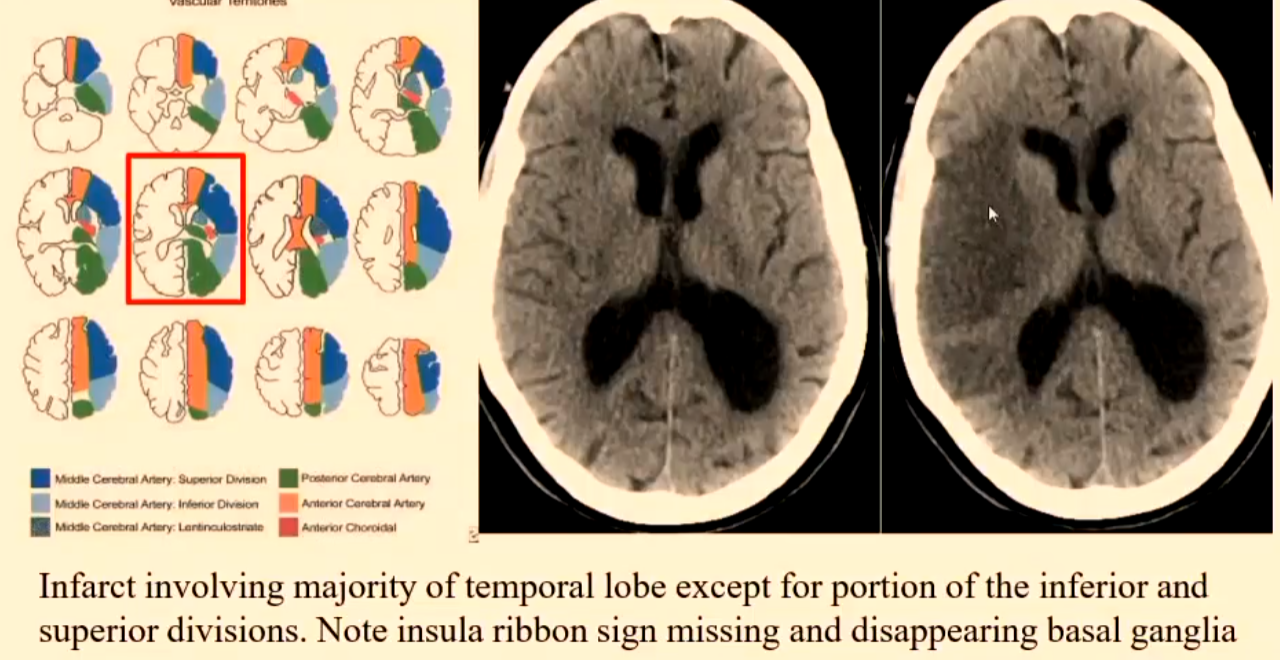
Right MCA Ischaemic Infarct
Superior and inferior divisions.
Infarct involving majority of temporal lobe except for portion of the inferior and superior divisions.
Note insula ribbon sign missing and disappearing basal ganglia.
Ischaemic Stroke
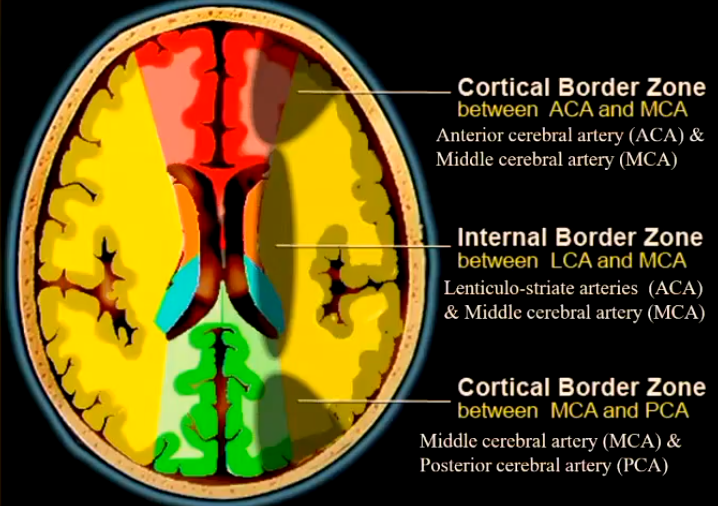
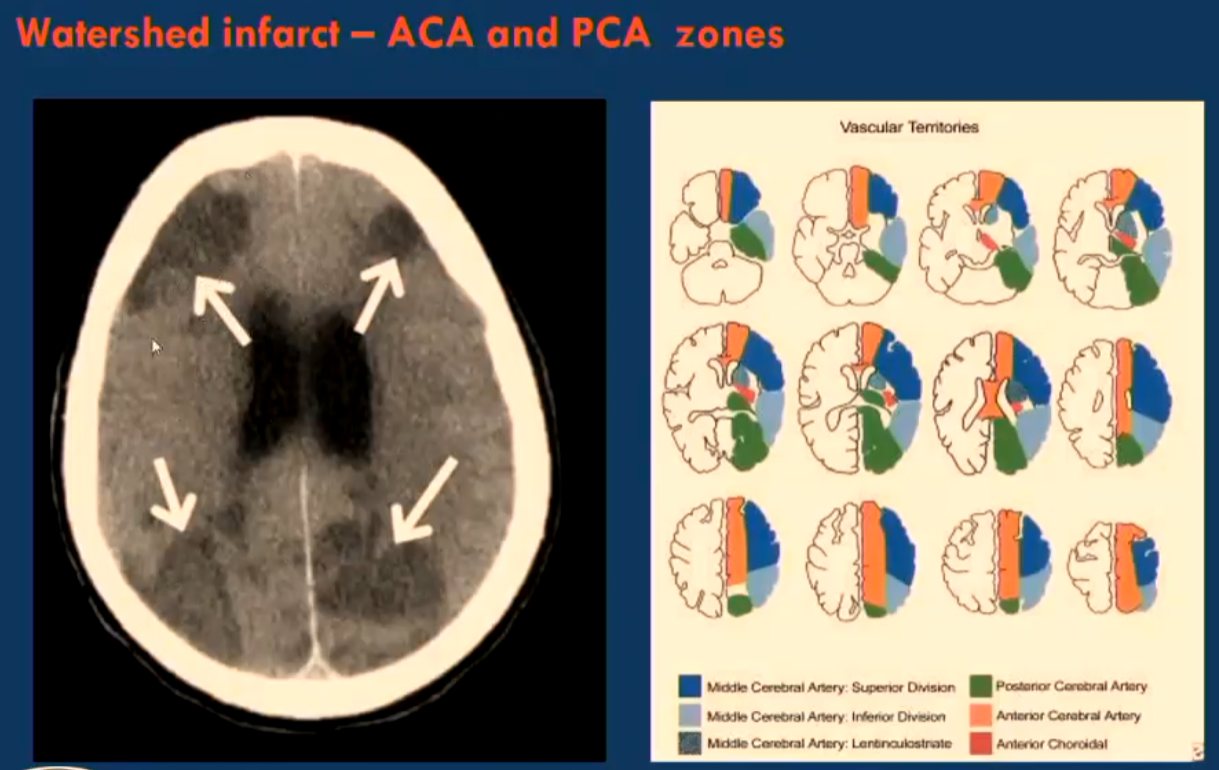
Watershed infarct or Border infarct
Ischaemic infarcts at the border between cerebral vascular territories which possess little or no anastomosis.
Anterior cerebral artery (ACA) & Middle cerebral artery (MCA)
Lenticulo-striate arteries (ACA) & Middle cerebral artery (MCA)
Middle cerebral artery (MCA) & Posterior cerebral artery (PCA)
Anastomosis: A connection between the anterior and posterior circulation of the brain, allowing for collateral blood flow in case of occlusion in one of the primary arterial systems.
Ischaemic Stroke
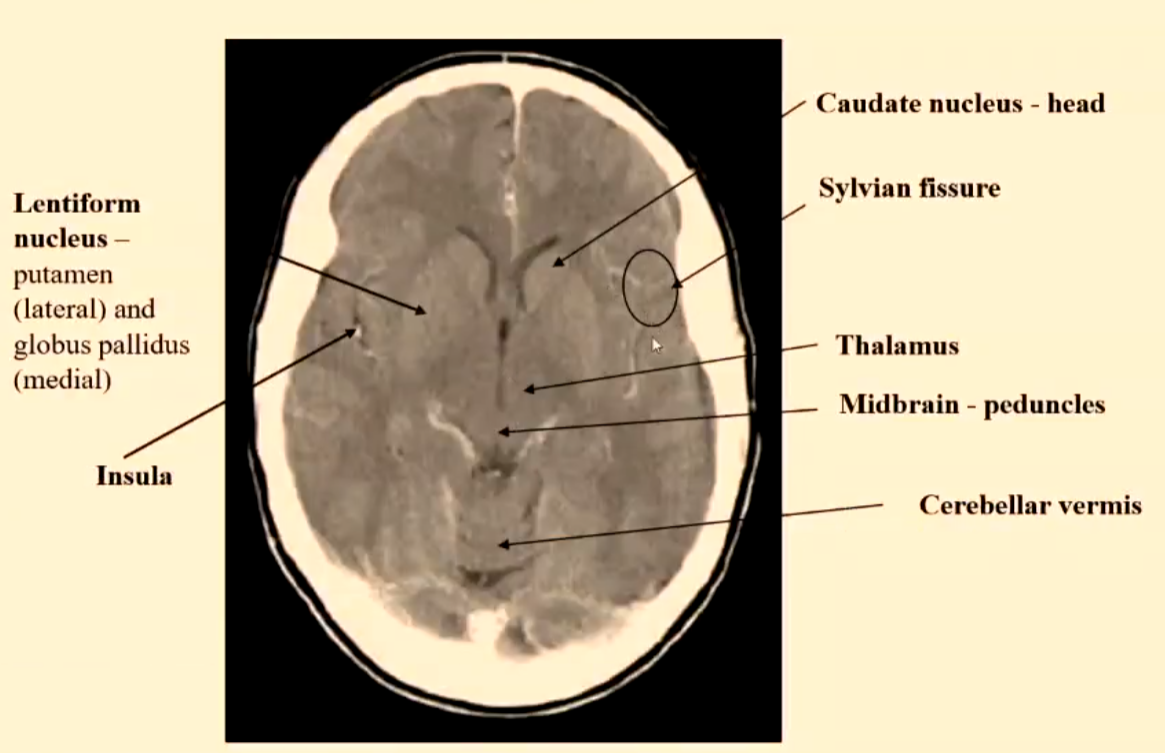
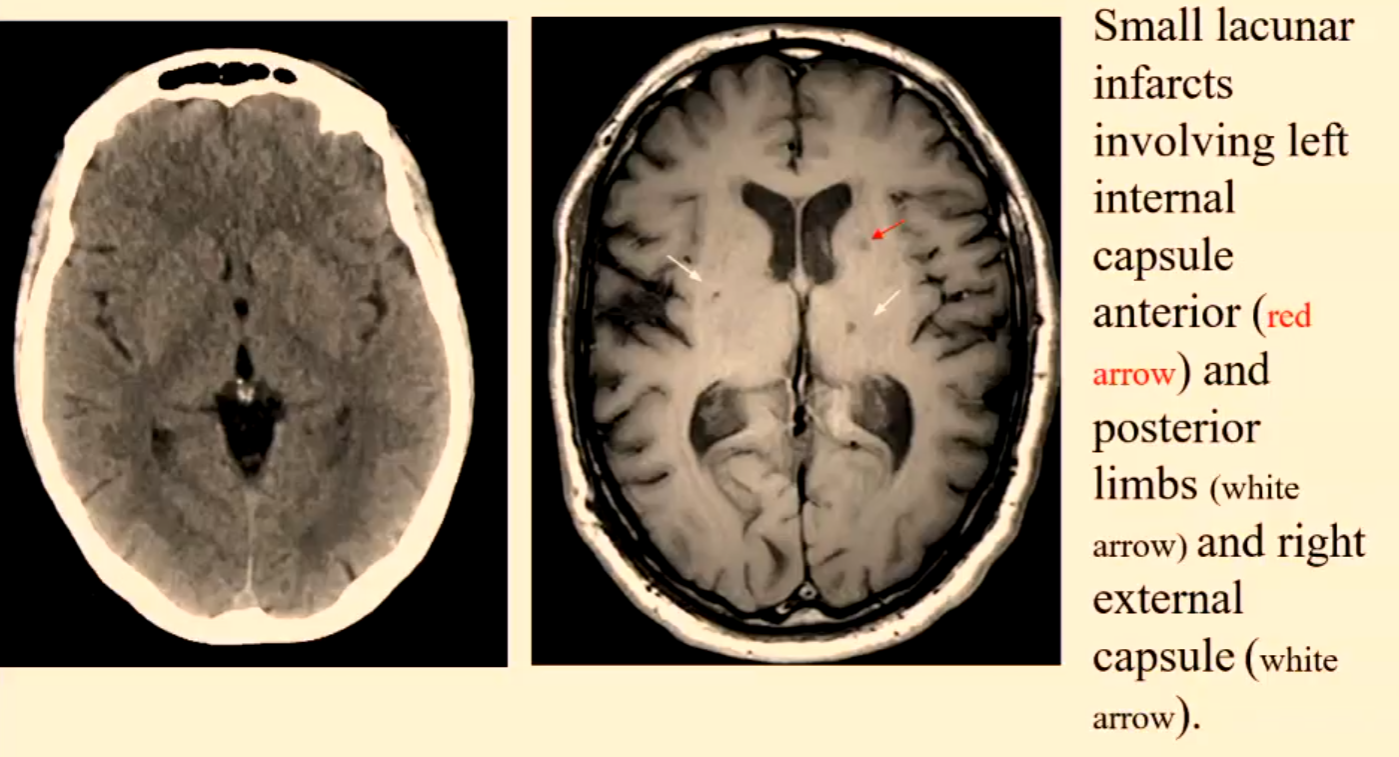
Lacunar/ lacuna infarct
Small <20mm ischaemic infarcts are due to microatheroma formation in small end penetrating arteries deep in the base of the brain.
Represent approx. 20% of cerebral infarctions.
Patients predisposed to having lacunar infarcts are hypertensive or diabetic patients.
Their inferior location is upper two-thirds of putamen or caudate nucleus or thalamus or pons or internal capsule
Modality for Detection of Intracranial Infarction (? Haemorrhagic Infarction or Intracranial Haemorrhage)
CT is accepted as the gold standard for imaging ? intracerebral haemorrhage or haemorrhagic infarction or post reperfusion of an ischaemic infarction
MR is at least as sensitive as CT in the detection of intracerebral haemorrhage and some authors maintain it is more sensitive during subacute or chronic phases and is equivalent to CT even in the hyperacute phase (within 6hrs of ictus).
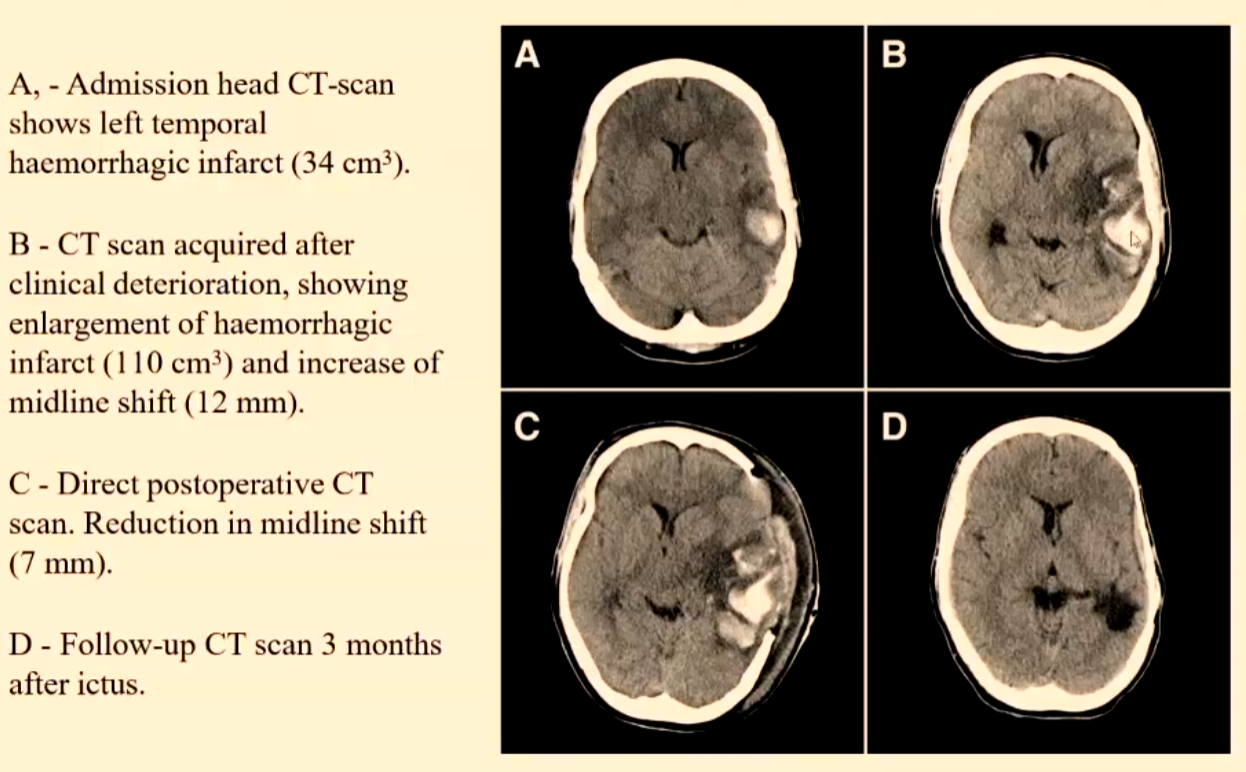
Imaging Modality for Detection of Intracerebral Haemorrhage
CT
It is important that the imaging can clearly distinguish an ischaemic stroke from a haemorrhagic stroke because of the divergent nature of their treatment (i.e. thrombolytic agent or Warfarin ARE NOT used in the management of a haemorrhagic stroke patient).
An hyperacute/acute haemorrhagic stroke will appear as HYPERDENSE on CT for a number of days due to the high protein concentration of haemoglobin and the retraction of the clot.
Over days the appearance on CT will become ISODENSE in the sub acute phase and then HYPODENSE in the chronic stage.
The progression thru the isodense and hypodense stages is due to the breakdown and clearing of the clot.
The initial appearances can be ISODENSE on a patient who is severely anaemic due to a low haematocrit < 20%.
MRI
With MRI the appearances change differently to CT. As the haematoma ages the oxyhaemoglobin breaks down into several paramagnetic products: deoxyhaemoglobin methaemoglobin finally, haemosiderin.
Imaging Modality for Determination of Tissue Viability
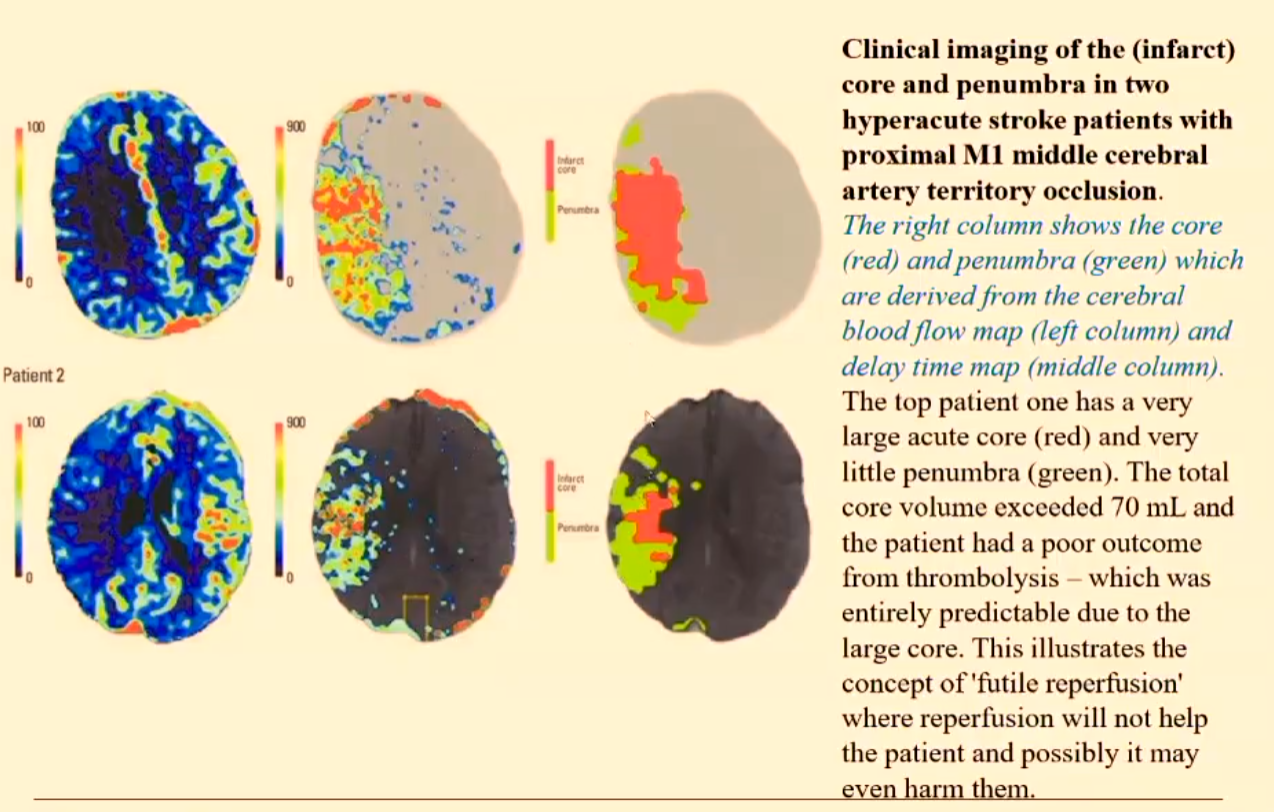
This is determining the ischaemic penumbra which is determining tissue viability and allows for the planning of the therapy and also extending the therapy time window.
MRI using DWI and PWI helps to identify brain tissue at risk of infarction.
Brain tissue will die within a very short time after there is a loss of blood supply – resulting in the CORE.
There will be surrounding tissue that may be dysfunctional but still viable - the ISCHAEMIC PENUMBRA.
The area of normal DWI signal but abnormal PWI signal is known as the perfusion-diffusion mismatch (PDM) and represents the ISCHAEMIC PENUMBRA.
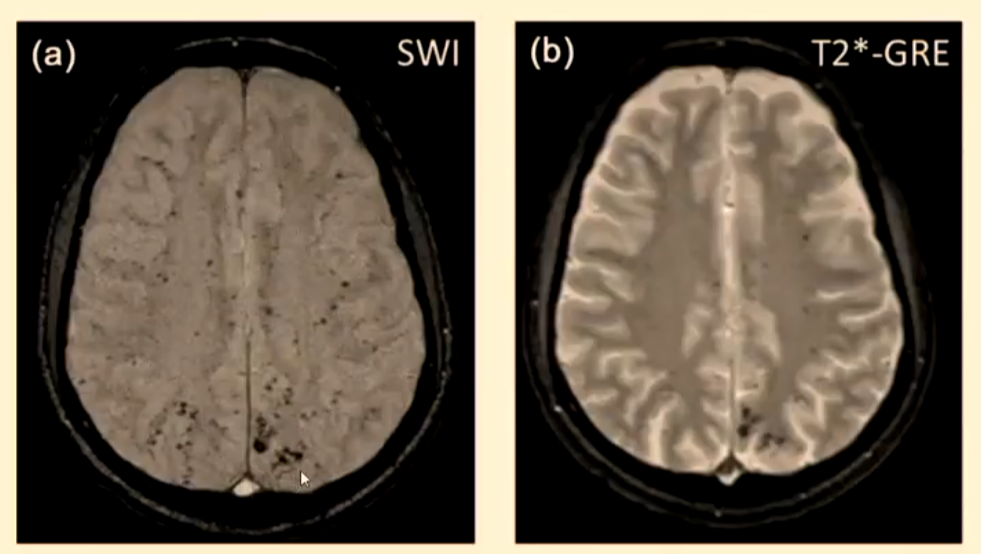
Microbleeds
It has been noted that cerebral microbleeds (CMBs) are becoming more frequent with ageing, patients with dementia and cerebrovascular disease.
This creates a problem with the patients who have suffered an ischaemic stroke and been treated with antiplatelet (thrombolytic agent) then having an intracerebral haemorrhage.
CMB are identified on MRI using susceptibility-weighted imaging (SWI) or T2*-GRE but are not visible on CT. This creates a problem with the hyperacute patient when there is a decision to be made about the use of thrombolysis.
Transient Ischaemic Attack
A TIA is defined as a sudden, focal neurological event of thrombo- embolic origin that lasts less than 24 hours.
Approximately, 25% of patients with an ischaemic stroke will have had 1 or more TIA event prior to the stroke
15% will have a stroke within 3 months and
50% will have a stroke within 48 hrs of the TIA.
Neuroimaging is critical for patients with TIA and MRI is the preferred and most sensitive modality for detecting the small defects from a TIA.
Neuroimaging is critical because the clinical diagnosis of a TIA is mainly determined by the clinical history as the neurological signs resolve after 24hrs distinct to a stroke.
MRI must include diffusion-weighted imaging (DWI) and this should be performed within 24hrs of the TIA.