Carbohydrates and Lipids
Course Overview
Course Title: Biology 189: Fundamentals for Life Sciences
Topics Covered: Carbohydrates and Lipids
Learning Objectives
Define essential terms:
Macromolecules: Large, complex molecules made of smaller parts called monomers.
Polymers: Made of repeating units (monomers).
Monomers: Simple building blocks of macromolecules.
Explain the formation and breakdown of polymers:
Dehydration Synthesis: chemical reaction to form covalent bonds between monomers
Hydrolysis: chemical reaction to break bonds and separate monomers

Define and understand carbohydrates and their roles:
Three primary functions in living organisms:
Energy source
Short-term energy storage
Structural support
Classify types of monosaccharides:
Monosaccharides: Single sugar molecules, crucial in various biological processes.
Recognize differences between:
Pentoses (5-carbon sugars)
Hexoses (6-carbon sugars)
Aldoses (sugars with aldehyde groups)
Ketoses (sugars with ketone groups)
Define glycosidic linkages and how monosaccharides join.
Differentiate larger carbohydrate types:
Disaccharides: Two monosaccharides joined.
Oligosaccharides: Few monosaccharides (3 to 10 units).
Polysaccharides: Many monosaccharides (hundreds or thousands).
Describe functions of key polysaccharides:
Starch: Energy storage in plants.
Glycogen: Energy storage in animal muscles and liver.
Cellulose: Provides structural support in plant cell walls.
Chitin: Found in arthropod exoskeletons and fungal cell walls.
Define lipids and their functions:
Three major types of lipids:
Triglycerides: Fats and oils for energy storage.
Phospholipids: Key components of cell membranes.
Steroids: Hormones and signaling molecules.
Distinguish between saturated, unsaturated, and trans fats.
Macromolecules Overview
Macromolecules: Large and complex molecules made from smaller units.
Polymers: Composed of repeating monomer units.
Polymer Formation and Breakdown
Dehydration Synthesis
Definition: Chemical reaction forming covalent bonds between monomers, resulting in water production.
Importance: Fundamental method of building macromolecules.
Hydrolysis
Definition: Reaction that breaks covalent bonds between monomers by adding water.
Application: Key process in digestion and metabolism for energy release.
Carbohydrates
Definition
Composition: Macromolecules made up of carbon, hydrogen, and oxygen. (C,H,O)
Functions:
Source of energy
Short-term energy storage.
Structural support for cells
Monosaccharides
Definition: Simplest form of carbohydrates (single sugar), composed of CnH2nOn.
Variations: Differ based on carbon number and arrangement of functional groups (-H and -OH).
Types of Monosaccharides
Pentoses: 5-carbon sugars (e.g., ribose).
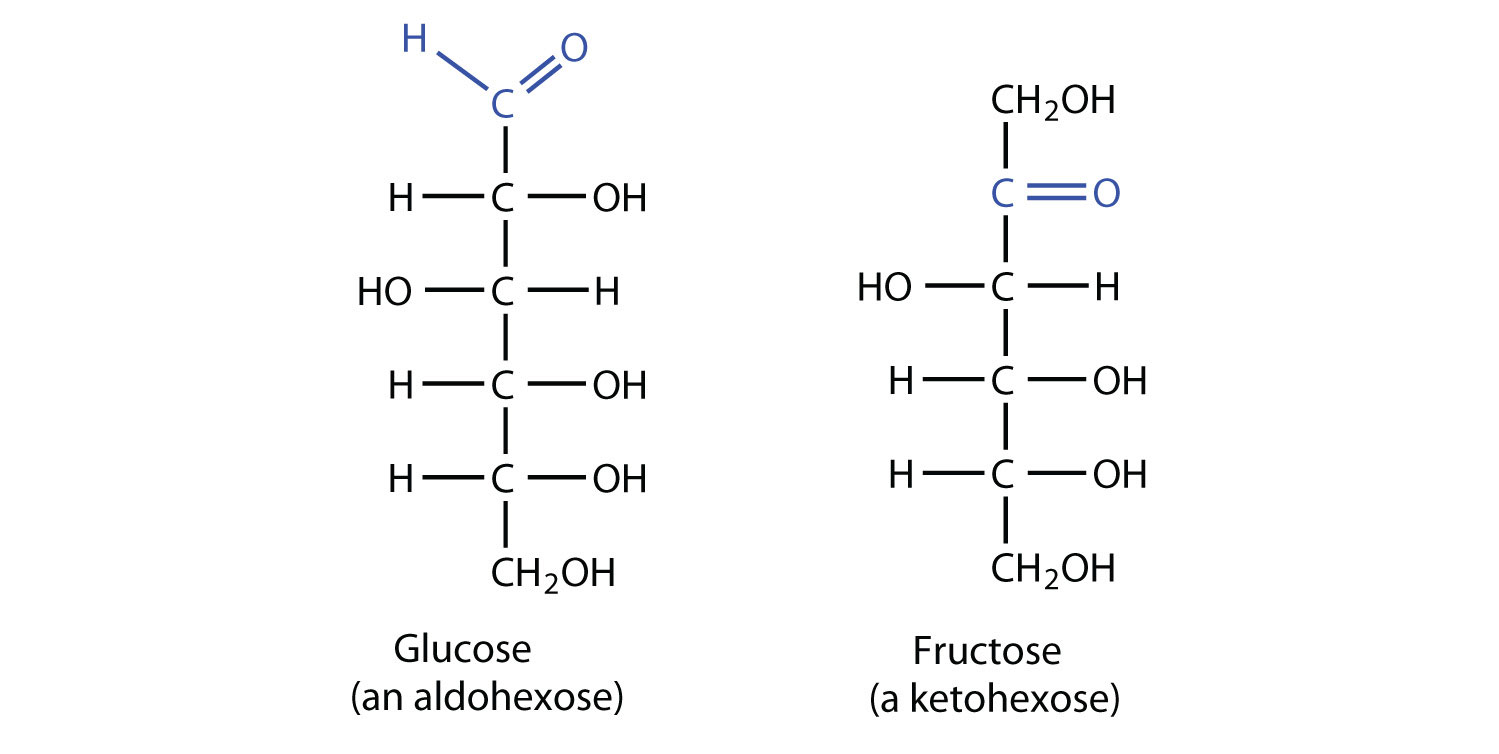
Hexoses: 6-carbon sugars (e.g., glucose, fructose, galactose).
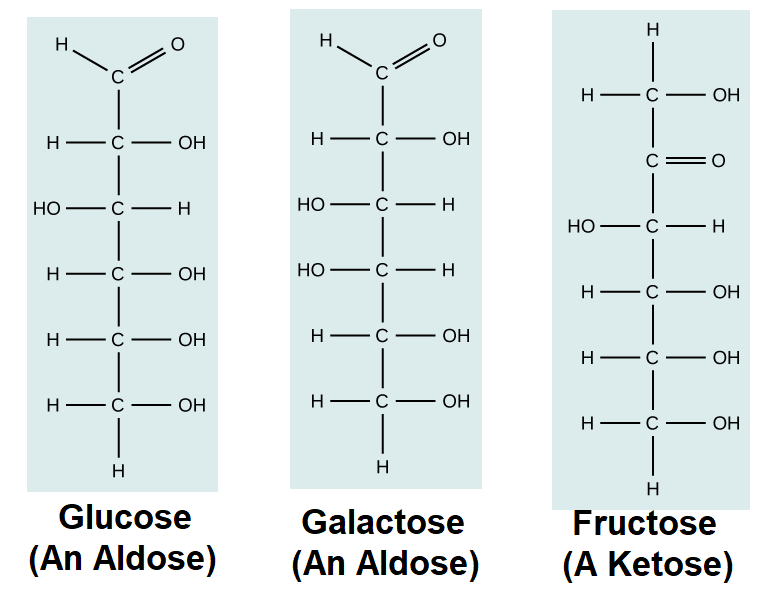
Aldose Characteristic: Sugars containing aldehyde group (e.g., glucose).
Ketose Characteristic: Sugars containing ketone group (e.g., fructose).
Glycosidic Linkage
Definition: Covalent bond formed between monosaccharides via a dehydration reaction.
Types of Carbohydrates:
Disaccharides: Two monosaccharides
Oligosaccharides: 3 to 10 (usually)
Polysaccharides: Many monosaccharides made of hundreds or more
Key Polysaccharides
Starch: Energy storage in plants; branched structure.
Glycogen: Energy storage in animals, highly branched.
Cellulose: is a polysaccharide is used for plant structure
Chitin: Found in exoskeletons of arthropods and fungal cell walls.
Lipids
Overview
Definition: Hydrophobic macromolecules primarily composed of C-H and C-C bonds, characterized by their nonpolar nature.
Types of Lipids:
Triglycerides: Consist of three fatty acids and glycerol for energy storage and insulation.
Phospholipids: Major component of cell membranes, amphipathic (both hydrophobic and hydrophilic parts).
Steroids: Structure consists of four fused rings, used in cell signaling (e.g., cholesterol, hormones).
Saturated and Unsaturated Fats
Saturated Fats: Only single C-C bonds; solid at room temperature.
Maximum hydrogen bonding leads to tight packing.
Unsaturated Fats: Contain one or more double bonds (kinks); liquid at room temperature due to loose packing.
Trans Fats: Created by hydrogenation, converting unsaturated fats to saturated fats.
Importance: Nutritional implications for health in dietary choices.
Functional Groups and Reactions
Triglycerides: Formed by ester linkages between fatty acids and glycerol.
Hydrolysis Reactions: Involved in breaking apart triglycerides for energy use.
Conclusion
Understanding carbohydrates and lipids is essential in biology, impacting energy storage, structural integrity, and cellular functions. Knowledge about macromolecules provides a groundwork for exploring complex biological processes.