DIFFUSION
Diffusion
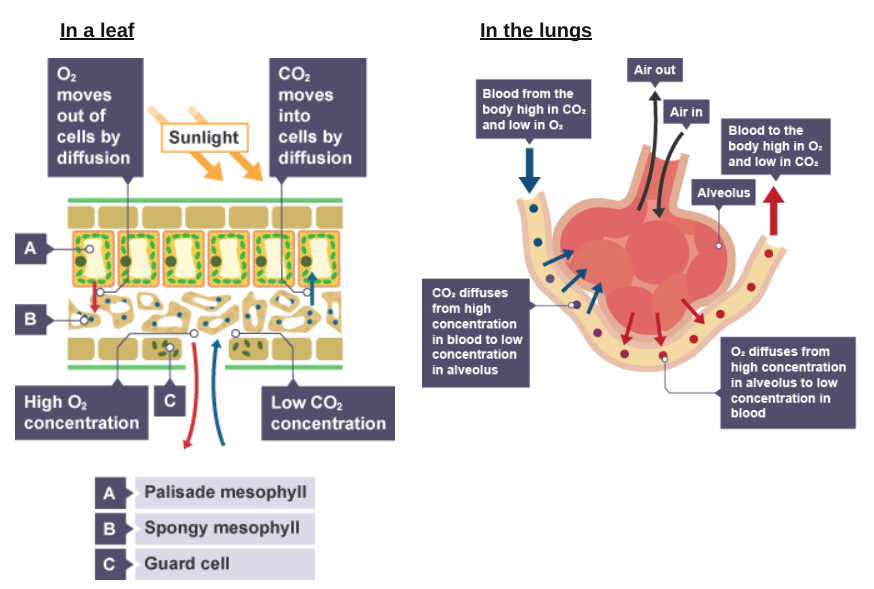
Particles (molecules and ions) in a liquid and a gas move continuously. Because of this movement, particles will spread themselves evenly throughout a liquid or a gas.
If there is a situation where particles of a substance are in a higher concentration, they will move from this region to where they are in a lower concentration. This is called diffusion.
It is important to remember that the particles:
will move in both directions, but there will be a net movement from high to low concentration
will end up evenly spread throughout the liquid or gas, but will continue to move
Some examples of diffusion in biological systems
Some substances move into and out of living cells by diffusion.