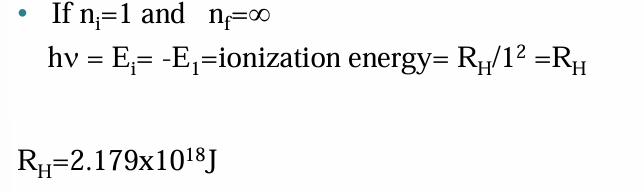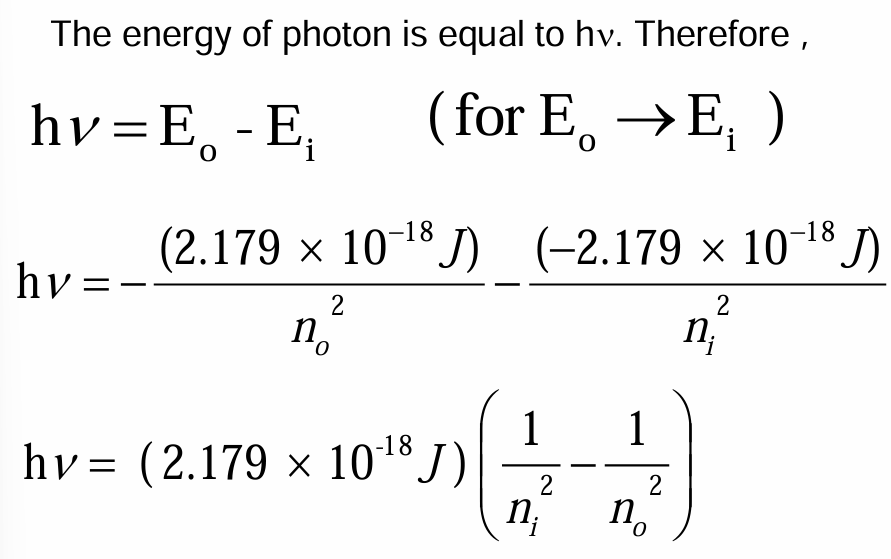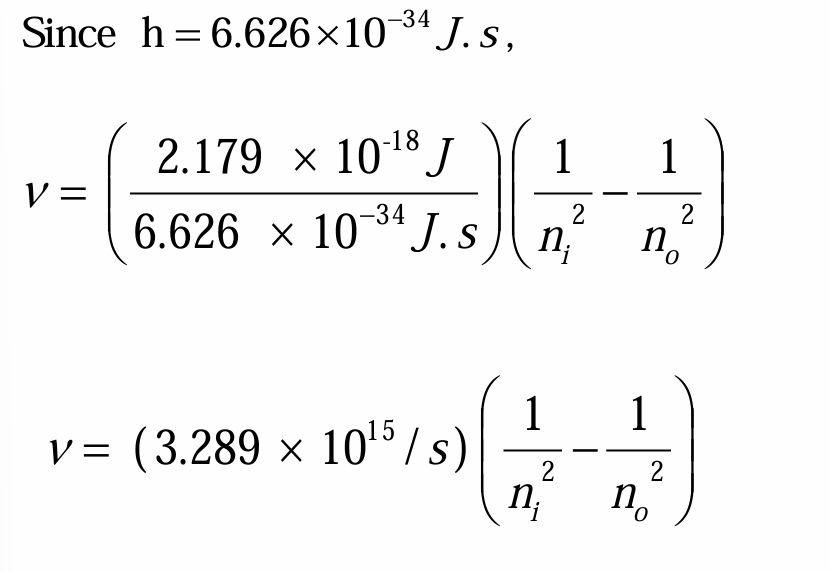Genel Kimya
LEWIS THEORY
Valence e- play a fundamental role in chemical bonding.
e- transfer leads to ionic bonds.
Sharing of e- leads to a covalent bond.
e- are transferred or shared to give each atom a noble gas configuration, the octet.
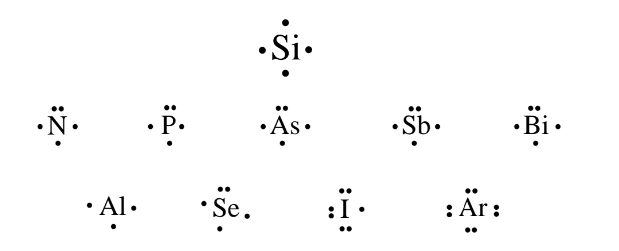
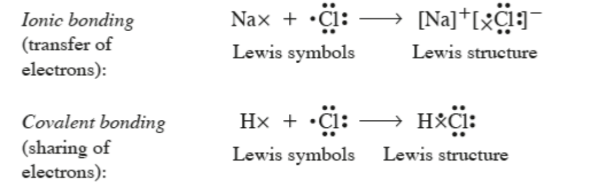
A central atom is bonded two or more atoms and a terminal atoms is bonded to just one other atom.
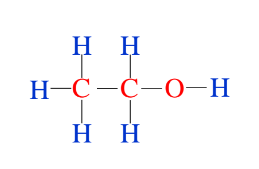
• Hydrogen atoms are always terminal atoms.
• Central atoms are generally those with the lowest electronegativity.
• Carbon atoms are always central atoms.
• Generally structures are compact and symmetrical.
Formal Charge
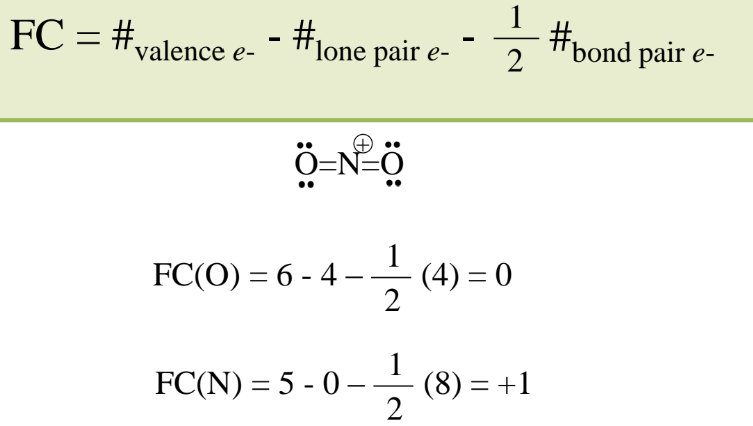
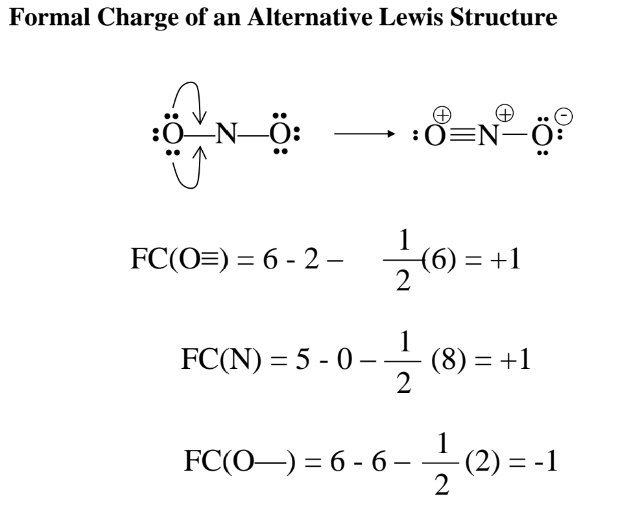
General Rules for Formal Charge
Sum of FC is the overall charge.
FC should be as small as possible.
Negative FC usually on most electronegative elements.
FC of same sign on adjacent atoms is unlikely.
Example:
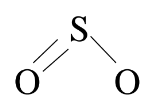
Diagram the Lewis structure of the SO2 molecule. The molecule is angular, and the two O atoms are bonded to a central S atom.
Solution:
1- Determine the total number of valence electrons that must appear in the structure. The total number of valence electron in SO2 is
6 e- (from the S atom)
+ 6 x 2=12e- (from the O atom)
18 e
2-Identify the central atom(s) and terminal atoms.
3-Determine the number of electrons that would be required to give 2 electrons to each H atom individually and 8 electrons to each of the other atoms individually.Since there are no H atoms in SO2
no.e- for individual atoms=2(no.H atoms)+8(no.other atoms)
=2(0)+8(3)=24e
4-The number obtained in step 3 minus the number obtained in step 1 is the number of electrons that must be shared in the final structure.
no.bonding e-=(no.e- for individual atoms)-(total no.electrons)
= 24-18 = 6 e
5-One-half the number of bonding electrons (step 4) is the number of covalent bonds in the final structure.
no.bonds=(no. bonding e- )/2
= 6/2 = 3
6-Write the symbols for the atoms represent in the structure,arranging them in the way that they are found in the structure.
7-The total number of electrons (step 1)minus the number of bonding electrons(step 4)is the number of unshared electrons.
no. unshared e-=(total no. e-)-(no. bonding e- )
=18-6=12 e
8-Indicate the formal charges of the atoms where appropriate.The formal charge of the S atoms is
formal charge =+(grup no.) - ( (no.bonds) + (no.unshared e)
=+ 6 - 3 -2 =1+
The formal charge of the left-hand O atom is
formal charge =+6 - 1- 6=1
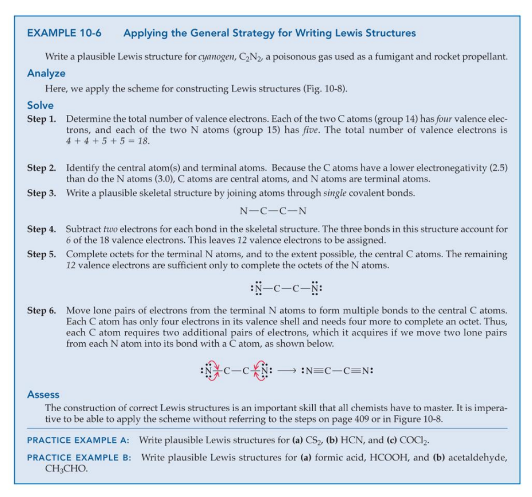

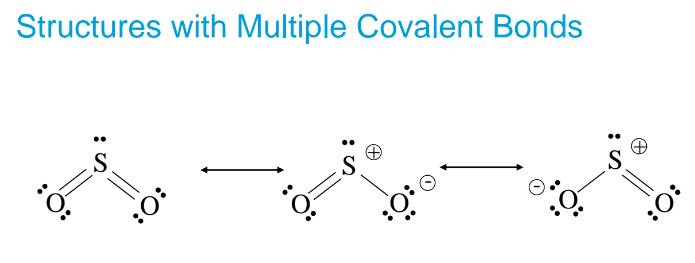
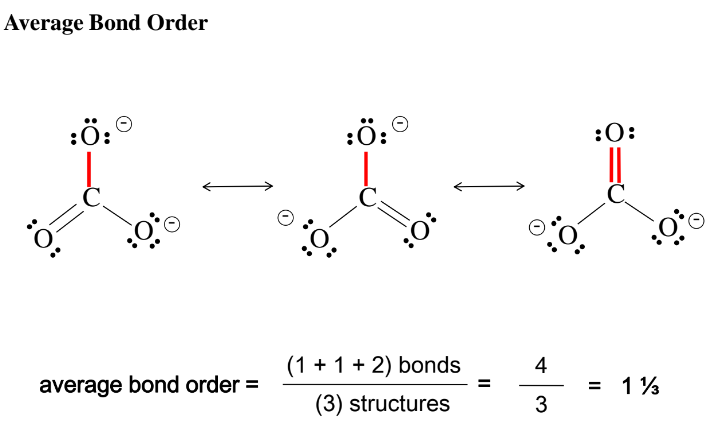
Resonance
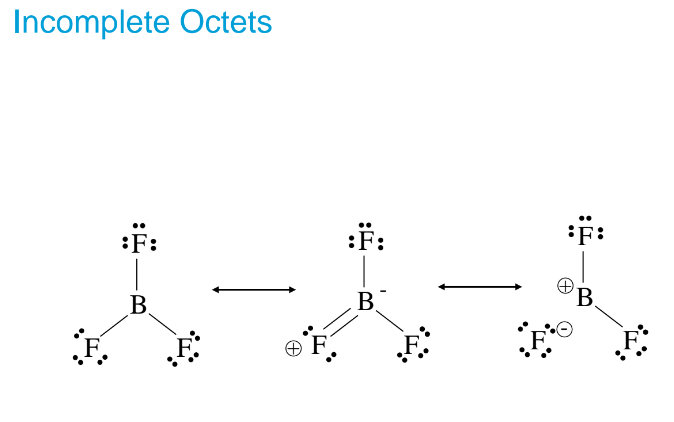
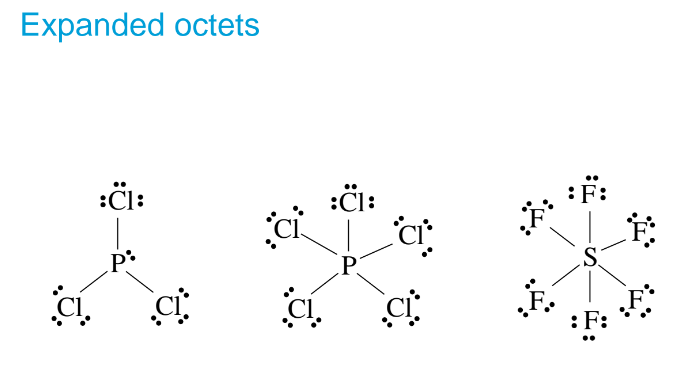
Exception of the Octet Rule
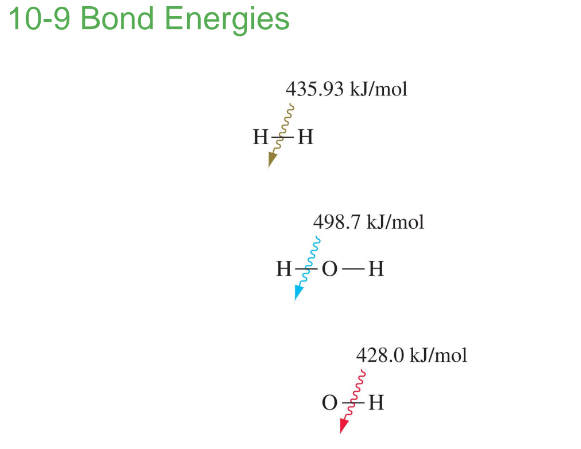
Bond length – distance between nuclei.
Bond angle – angle between adjacent bonds.
VSEPR Theory
Electron pairs repel each other whether they are in chemical bonds (bond pairs) or unshared (lone pairs). Electron pairs assume orientations about an atom to minimize repulsions.
Electron group geometry – distribution of e- pairs.
Molecular geometry – distribution of nuclei.
Applying VSEPR Theory
1. Draw a plausible Lewis structure.
2. Determine the number of e- groups and identify them as bond or lone pairs.
3. Establish the e- group geometry.
4. Determine the molecular geometry.
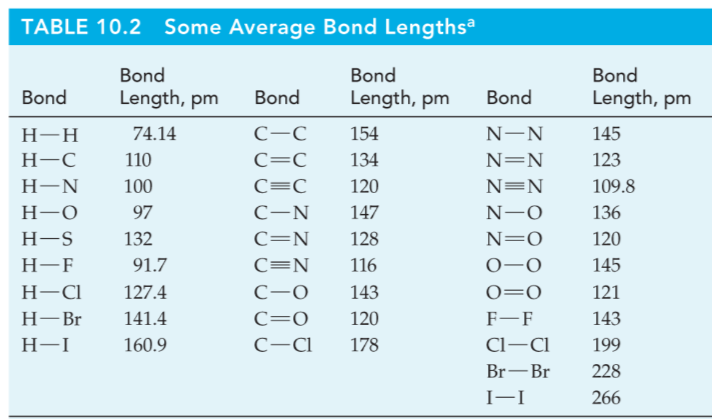
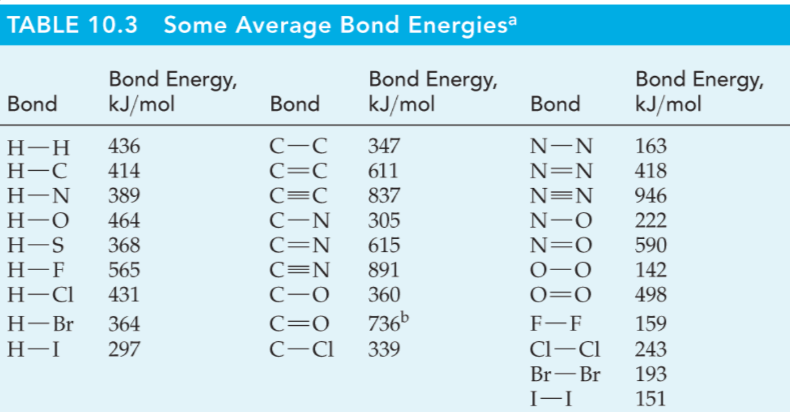
Bond Order and Bond Length
Bond Order
Single bond, bond order = 1
Double bond, bond order = 2
Triple bond, bond order = 3
Bond Length
The distance between the centers of two atoms joined by a covalent bond.
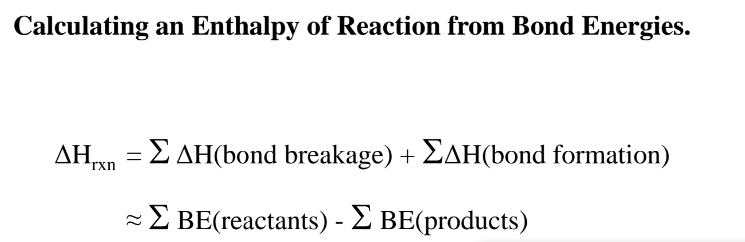
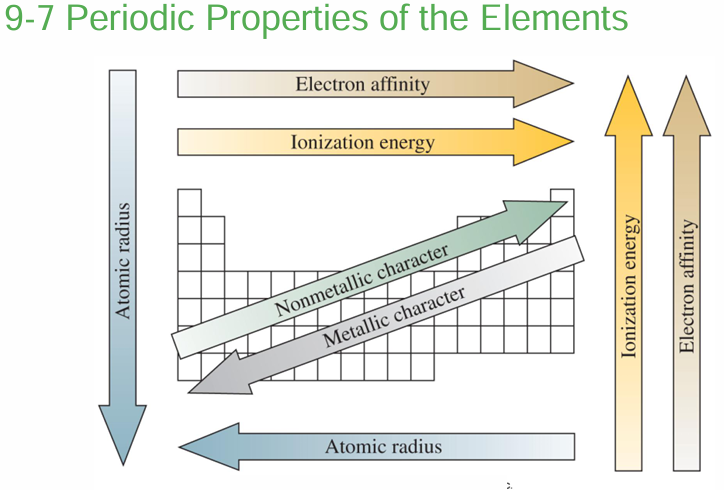
PERIODIC TABLE
1869 - Dimitri Mendeleev - Lothar Meyer
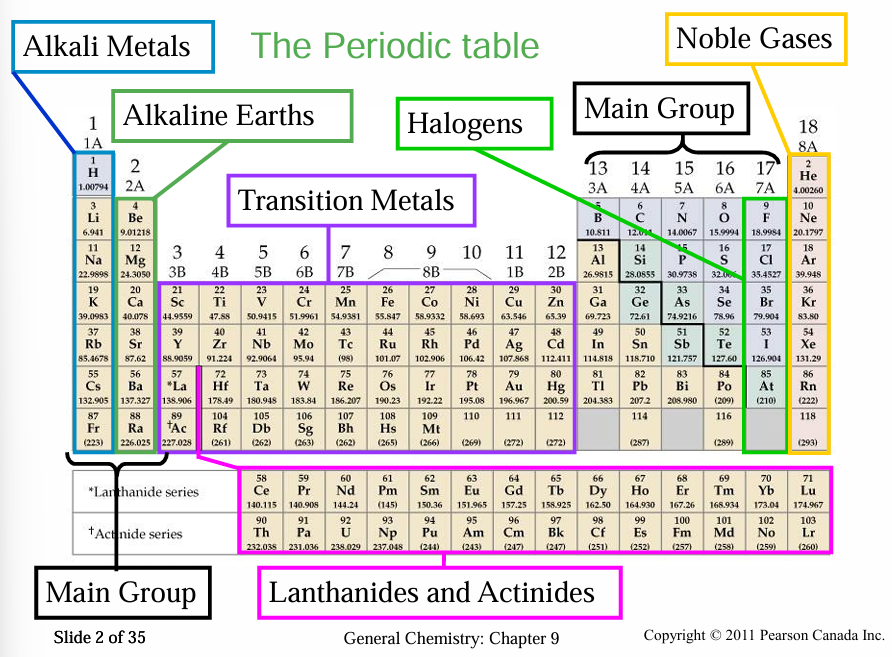
Metals and Nonmetals and Their Ions
Metals
Good conductors of heat and electricity.
Malleable and ductile.
Moderate to high melting points.
Nonmetals
Nonconductors of heat and electricity.
Brittle solids.
Some are gases at room temperature.
Metalloids
Metallic and non-metallic properties
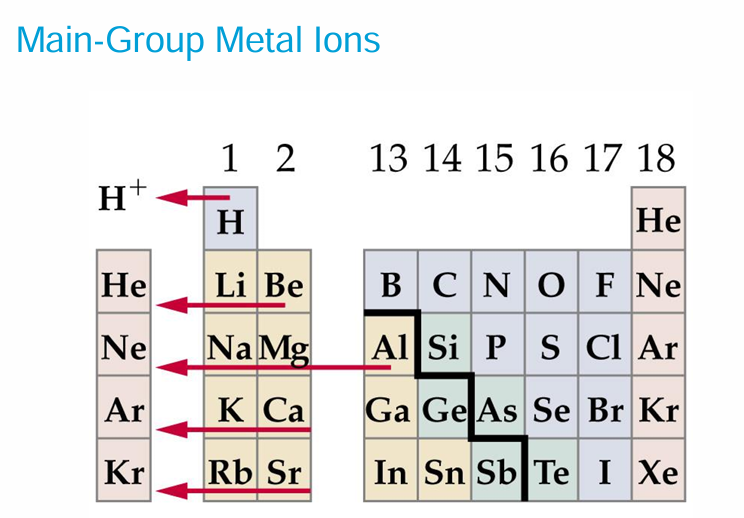
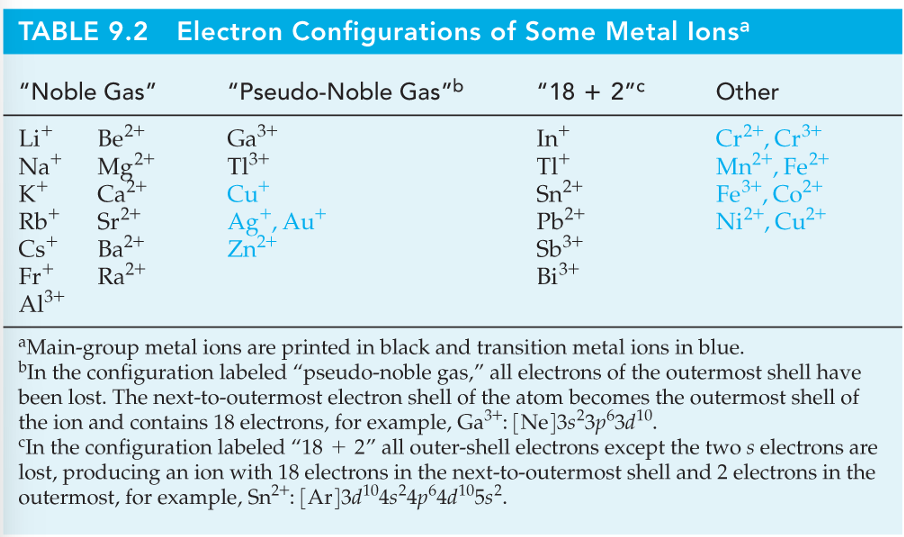
• Not all stable ions result in the noble gas configuration; there are a few exceptions mainly in the transition metals. Zn 1s2 2s2 2p6 3s2 3p6 3d10 4s2 loses the two valence electrons to become Zn 2+ 1s2 2s2 2p6 3s2 3p6 3d10 that is stable but does not have the configuration of a noble gas. It does have a complete valence shell. This is known as the pseudo noble gas electron configuration. Other ions like Cu+, Ag+, Au+ and Cd 2+ have pseudo noble gas configurations.
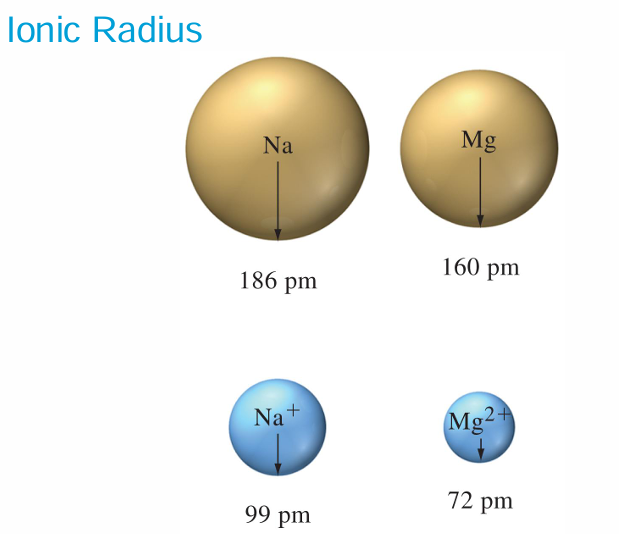
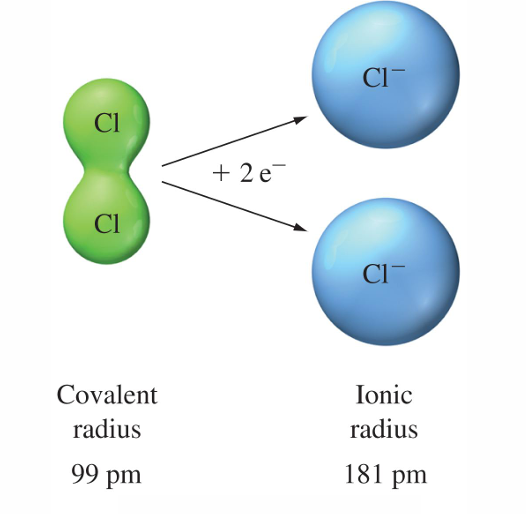
Cations are smaller than the atoms from which they are formed.
Anions are larger than the atoms from which they are formed.
• Na+1 and Mg +2 are isoelectronic- they have equal numbers of electrons (10) in identical configuration. Mg +2 is smaller than Na+1
For isoelectronic cations, the more positive the ionic charge, the smaller the ionic radius.
For isoelectronic anions, the more negative the charge, the larger the ionic radius.

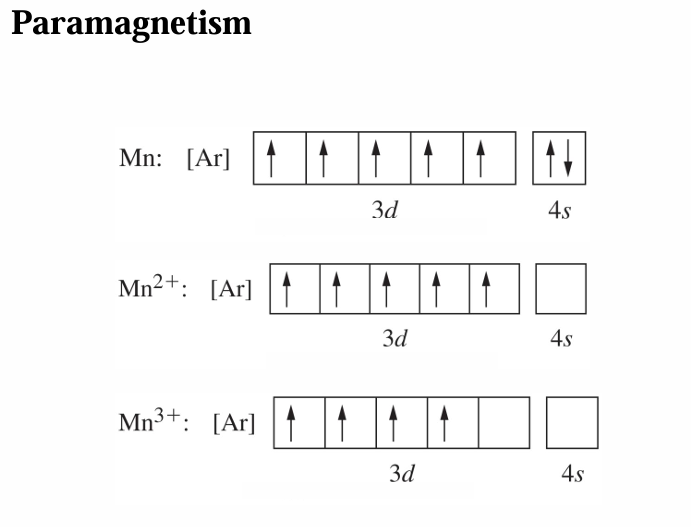
Magnetic Properties
Diamagnetic atoms or ions:
All e- are paired.
Weakly repelled by a magnetic field.
Paramagnetic atoms or ions:
Unpaired e-.
Attracted to an external magnetic field.

Electrons in Atoms
• We examine two important models—the Bohr model and the quantum-mechanical model—that propose explanations for the inertness of helium, the reactivity of hydrogen, and the periodic law.
• These models explain how electrons exist in atoms and how those electrons affect the chemical and physical properties of elements.
Niels Bohr and Erwin Schrödinger, along with Albert Einstein, played a role in the development of quantum mechanics.
Electromagnetic Radiation
Light is a form of electromagnetic radiation. Light is a type of energy that travels through space at a constant speed of 3.0 x 10^8 m/s . Light has properties of both waves and particles.
Light: Electromagnetic Radiation
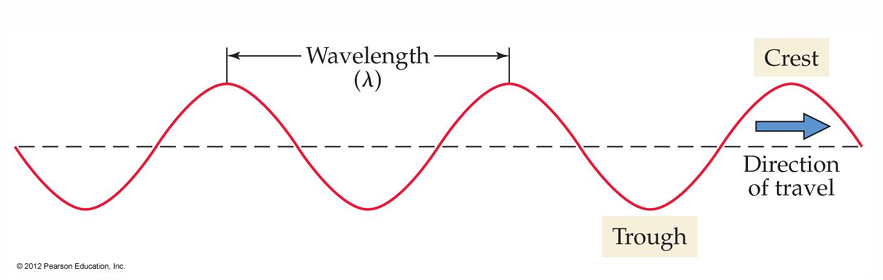
• Wavelength: The wavelength of light, λ (lambda, pronounced “lam-duh”), is defined as the distance between adjacent wave crests.
Light: Color of Light
• Red light, with a wavelength of 750 nm (nanometers), has the longest wavelength of visible light.
• Violet light, with a wavelength of 400 nm, has the shortest wavelength of visible light (1 nm = 1 x 10-9 m).
Components of white light
R O Y G B I V
• Light is separated into its constituent colors—red, orange, yellow, green, blue, indigo, and violet—when it is passed through a prism.
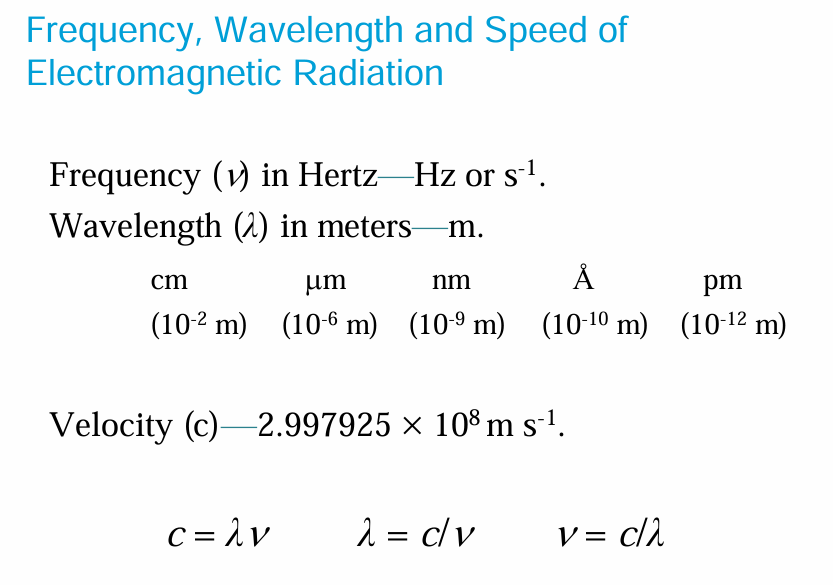
• Frequency: The frequency of light, ν (nu) is defined as the number of cycles or crests that pass through a stationary point in one second.
• Wavelength and frequency are inversely related—the shorter the wavelength, the higher the frequency.
• A particle of light is called a photon.
The wavelength of electromagnetic radiation determines the amount of energy carried by one of its photons.
The shorter the wavelength, the greater the energy of each photon. The frequency and energy of electromagnetic radiation are inversely related to its wavelength.
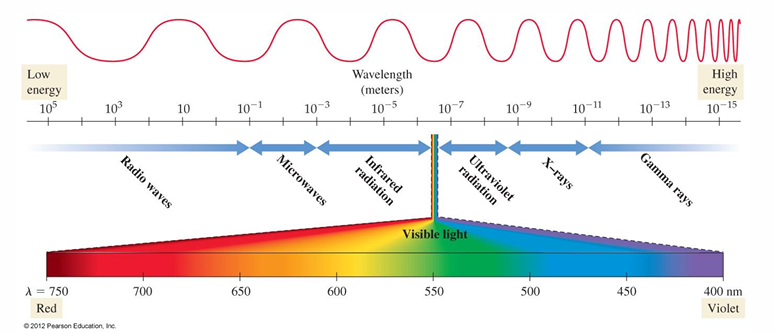
• The shortest wavelength and most energetic photons are those of gamma rays.
• Gamma rays are produced by the sun, by stars, and by certain unstable atomic nuclei on Earth.
• Next on the electromagnetic spectrum, with longer wavelengths and lower energy than gamma rays, are X-rays, familiar to us from their medical use.
• X-rays pass through many substances that block visible light and are used to image internal bones and organs.
• Between X-rays and visible light in the electromagnetic spectrum is ultraviolet or UV light, familiar to us as the component of sunlight that produces a sunburn or suntan.
• While not as energetic as gamma-ray or X-ray photons, ultraviolet photons still carry enough energy to damage biological molecules.
• Next on the spectrum is visible light, ranging from violet (shorter wavelength, higher energy) to red (longer wavelength, lower energy).
• Photons of visible light do not damage biological molecules.
• Infrared light is next, with even longer wavelengths than visible light.
• The heat you feel when you place your hand near a hot object is infrared light.
• All warm objects, including human bodies, emit infrared light.
• Beyond infrared light, at longer wavelengths still, are microwaves, used for radar and in microwave ovens.
• Microwave light has longer wavelengths—and therefore lower energy per photon—than visible or infrared light.
• Microwave light is efficiently absorbed by water and can heat substances that contain water.
• Substances that contain water, such as food, are warmed by the radiation of a microwave oven, but substances that do not contain water, such as a plate, are not.
• The longest wavelengths of light are radio waves, which are used to transmit the signals used by AM and FM radio, cellular telephones, television, and other forms of communication.
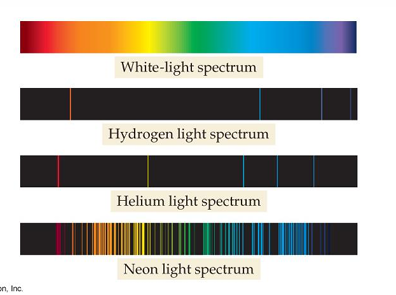
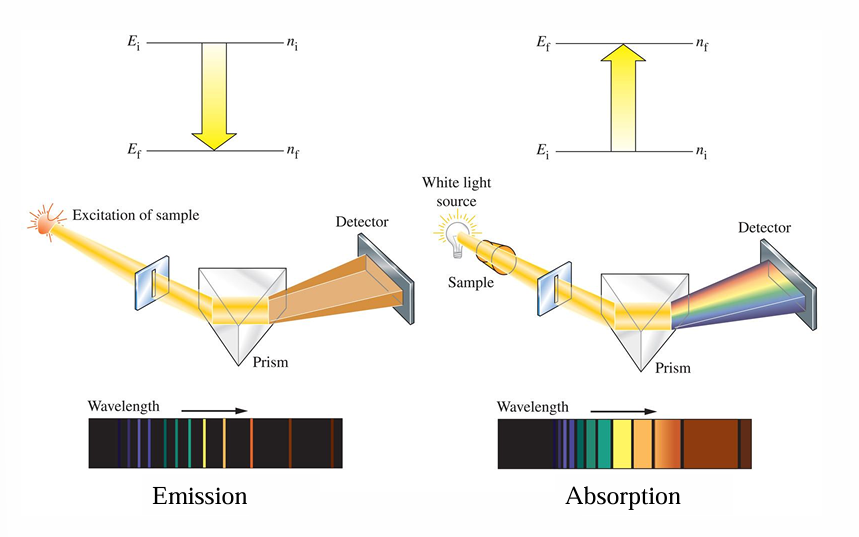
A white-light spectrum is continuous, with some radiation emitted at every wavelength.
The emission spectrum of an individual element includes only certain specific wavelengths.
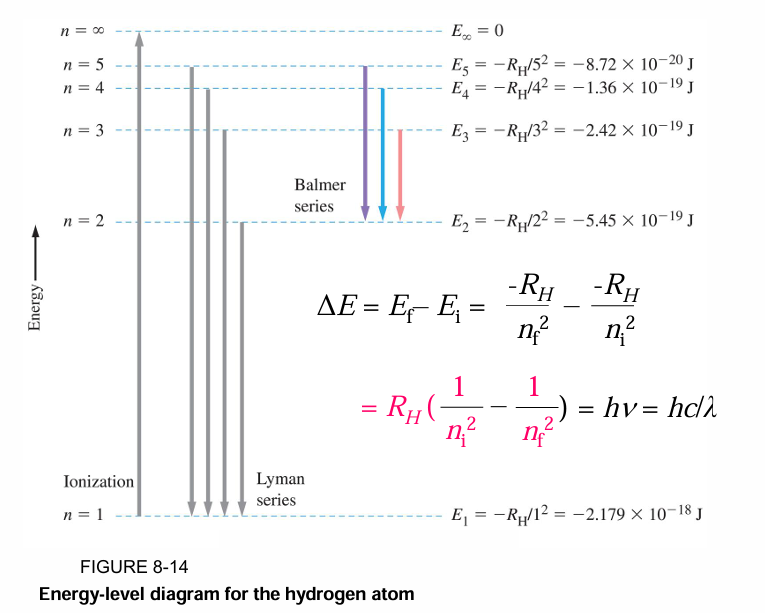
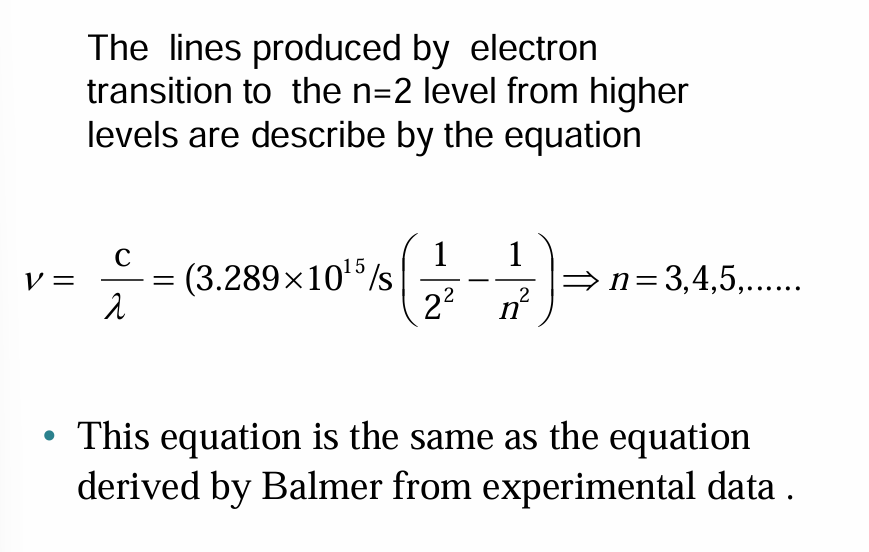
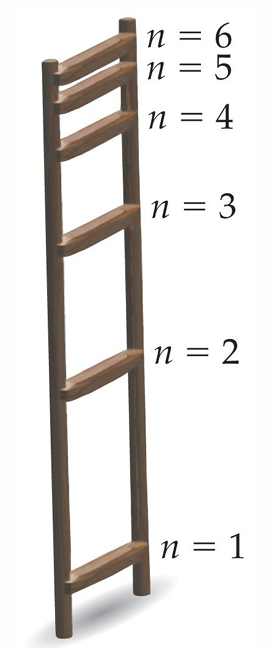
The energy of each Bohr orbit, specified by a quantum number n = 1, 2, 3 is fixed, or quantized. Bohr orbits are like steps of a ladder, each at a specific distance from the nucleus and each at a specific energy. It is impossible for an electron to exist between orbits in the Bohr model.
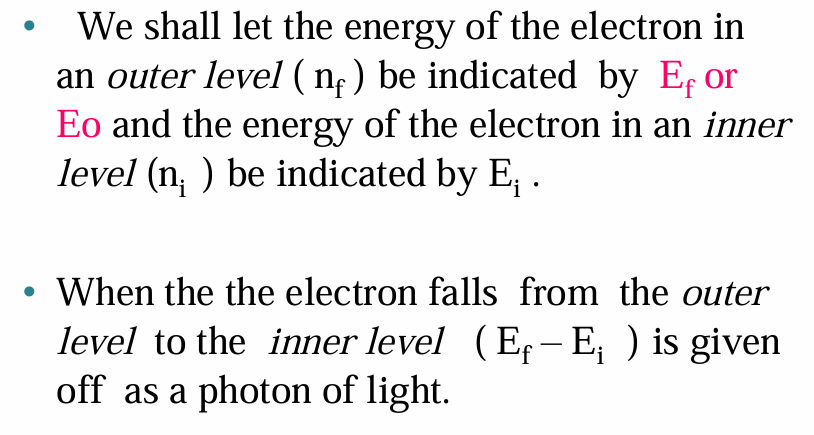
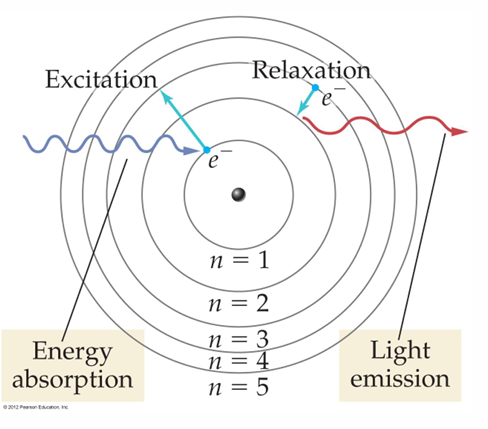
• When a hydrogen atom absorbs energy, an electron is excited to a higher-energy orbit. The electron then relaxes back to a lower-energy orbit, emitting a photon of light.
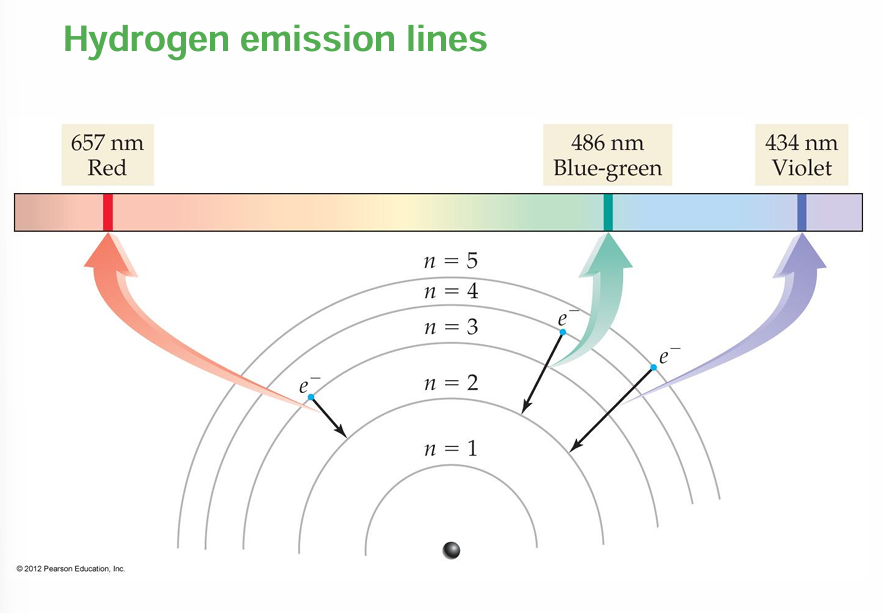
Hydrogen emission lines
• Since the amount of energy in a photon is directly related to its wavelength, the photon has a specific wavelength.
• The light emitted by excited atoms consists of specific lines at specific wavelengths, each corresponding to a specific transition between two orbits.
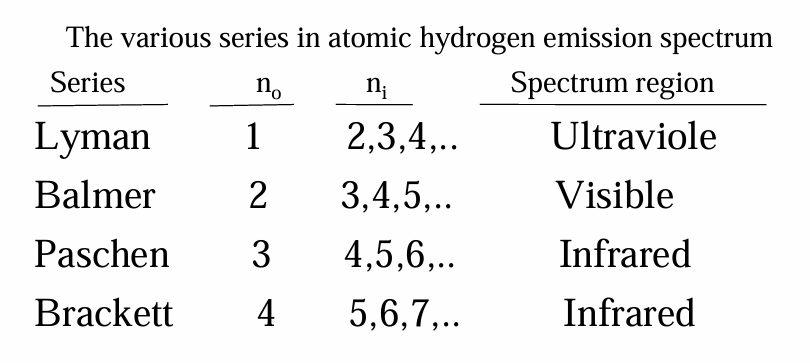
• For example, the line at 486 nm in the hydrogen emission spectrum corresponds to an electron relaxing from the n = 4 orbit to the n = 2 orbit.
• In the same way, the line at 657 nm (longer wavelength and lower energy) corresponds to an electron relaxing from the n = 3 orbit to the n = 2 orbit.
The Bohr Theory and the Iyonization Energy of Hydrogen
• The Bohr model of the atom helps the clarify the mechanism of formation of cations. In the special case where the energy of a photon interacting with a hydrogen atom is just enough to remove an electron from the ground state (n=1),the electron is freed, the atom is ionized,and the energy of the free electron is zero.