Note
5.0(1)
Chat with Kai
Explore Top Notes Note
Note Studied by 27 people
Studied by 27 people Note
Note Studied by 935 people
Studied by 935 people Note
Note Studied by 1 person
Studied by 1 person Note
Note Studied by 11 people
Studied by 11 people Note
Note Studied by 32 people
Studied by 32 people Note
Note Studied by 14 people
Studied by 14 people
Chapter 28: The Aggregate Expenditures Model
5.0(1)
How to cite stimulus materials in AP Seminar
5.0(2)
The Mirror Neuron System
5.0(1)
Animal Studies of Attachment
5.0(1)
English Tenses With Examples And When It Should Be Used
5.0(2)
Monetary Policy Notesheet
5.0(1)
4.2 Electron Transport Chain
Electrons (with their hydrogens) are passed from one molecule to the next in order of increasing electronegativity
Energy is released with each transfer
O2 is the final electron acceptor
This energy is used to pump H+ across from matrix to intermembrane space, setting up an electrochemical gradient of protons
- Proton gradient
- Proton-motive force (back down to low electronegativity through ATP synthase)
- Will be used to form ATP through OP
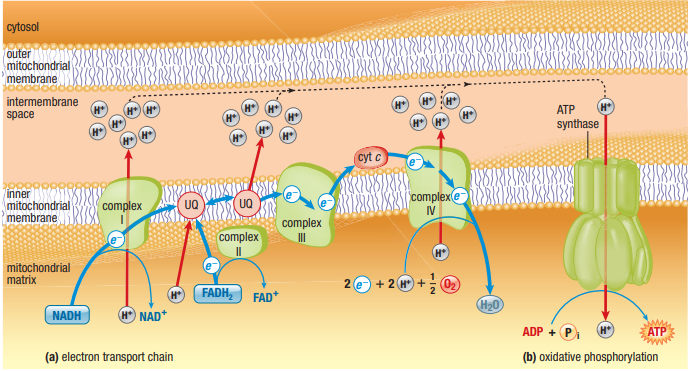
IN DETAIL
- 1st Oxidation
- NADH dehydrogenase oxdized NADH to NAD+
- Energy released drives H+ from matrix into intermembrane space
- 2nd & 3rd Oxidation
- Succinate dehydrogenase oxdizes FADH2
- Ubiquinone oxidizes NADH dehydrogenase ans Succinate dehydrogenase
- UQ is hydrophobic, shuttles within bilayer
- uses electrons to move H+ across membrane
- 4th & 5th Oxidation
- Cytochrome complex oxidizes UQ
- Cyt C oxidizes Cytochrome complex
- cyt c is hydrohphillic, shuttles in the intermembrane space
- 6th & 7th Oxidation
- Cytochrome oxidase oxidizes Cyt C
- Oxygen oxidizes cytochrome oxidase
- energy released pumps H+ across the membrane
- 8th Reaction
- Proton gradient sets up proton-motive force
- only way back is with ATP synthase
- Flow of protons allows the energy to synthesize ATP
- Chemiosmosis
- OP
Note
5.0(1)
Chat with Kai
Explore Top Notes Note
Note Studied by 27 people
Studied by 27 people Note
Note Studied by 935 people
Studied by 935 people Note
Note Studied by 1 person
Studied by 1 person Note
Note Studied by 11 people
Studied by 11 people Note
Note Studied by 32 people
Studied by 32 people Note
Note Studied by 14 people
Studied by 14 people
Chapter 28: The Aggregate Expenditures Model
5.0(1)
How to cite stimulus materials in AP Seminar
5.0(2)
The Mirror Neuron System
5.0(1)
Animal Studies of Attachment
5.0(1)
English Tenses With Examples And When It Should Be Used
5.0(2)
Monetary Policy Notesheet
5.0(1)