CHEM-LAB (4)
Experiment No. 6: Properties of Alcohols and Phenols
Intended Learning Outcomes
Familiarization with physical characteristics and properties of alcohols and phenols.
Differentiation between primary, secondary, and tertiary alcohols, and phenols through their physical and chemical characteristics.
Apparatus and Equipment
Test tubes
Evaporating dish
Funnel
Test tubes rack
Iron stand/tripod
Beaker
Test tube holder
Iron ring
Burner
Wire gauze
Materials
Ethyl alcohol
Concentrated HCl
6M NaOH
Isopropyl alcohol
ZnCl2
Iodine in KI solution
Tert-butyl alcohol
10% K2Cr2O7
Theoretical Background
Alcohols and Phenols: Important organic compounds, used in industrial and pharmaceutical applications. Ethanol is a well-known alcohol produced through fermentation.
Structure: Contain the hydroxyl functional group (-OH).
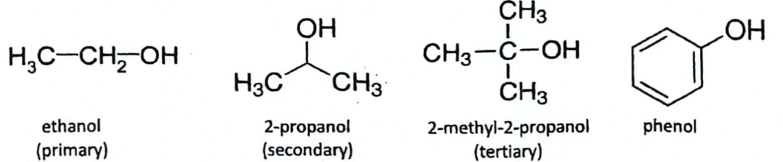
Classification of Alcohols:
Primary Alcohol: Attached to one carbon atom, two hydrogens; e.g., CH3-OH (ethanol).
Secondary Alcohol: Attached to two carbon atoms, one hydrogen; e.g., 2-propanol.
Tertiary Alcohol: Attached to three carbon atoms; e.g., 2-methyl-2-propanol.
The use of R, R’ and R’’’ signifies that each of those alkyl groups my be different.
Properties of Phenols
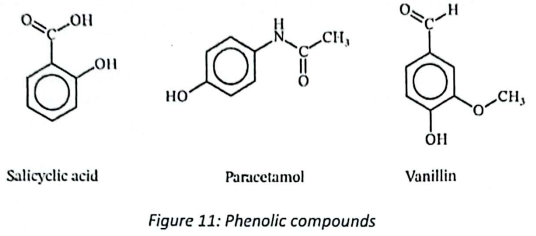
Phenol (C6H5OH): Colorless solid, low melting point, medicinal odor, dissolves in water, known as carbolic acid.
Weak Acid: Can denature proteins; must be handled cautiously.
Uses: Antiseptic and disinfectant in dilute solutions.
Caution: Toxic; can cause skin burns. Safety gear required.
Chemical Properties of Alcohols
A. Bordwell-Wellman's Test
Test Solution: Potassium dichromate (K2Cr2O7) in sulfuric acid (H2SO4) – orange-yellow color due to dichromate ion.
Reactants:
Orange-yellow solution: dichromate (Cr2O7^2-) ion.
The oxidation number of chromium in dichromate is reduced to chromic ion (Cr3+)
Reaction Products:
Dichromate is converted to chromic oxide (Cr2O3) and the blue-green solution is due to the chromic ion.
Oxidation Process: Primary alcohols oxidized to carboxylic acids, secondary to ketones, tertiary alcohols do not react (without breaking the molecule’s C-C bonds). Change from orange-yellow to blue-green indicates presence of alcohols.
The primary alcohol is oxidized first to aldehyde which will eventually oxidized to an acid.
Phenols Reaction: Can also be oxidized, yielding different products; same color change observed.
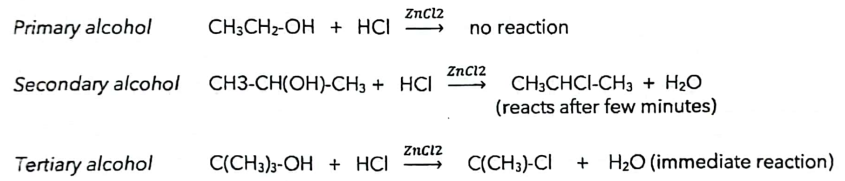
B. Lucas Test for Primary, Secondary, Tertiary Alcohols
Reagent: ZnCl2 in hydrochloric acid.
Reaction Rates:
Primary alcohols: No reaction.
Secondary alcohols: Cloudy in 5-10 minutes, may require heating.
Tertiary alcohols: Immediate reaction, vigorous and distinct color change.
Procedure for Bordwell-Wellman Test
Place 1 mL each of unknowns A, B, C, D in separate test tubes.
Add 5 drops of K2Cr2O7 and dilute sulfuric acid to each.
Warm in a water bath; observe color, odor, and oxidation rate.
Identify unknowns based on reactions.
Record results in the observation table.
Procedure for Lucas Test
Place 1 mL of unknowns A, B, C in separate test tubes.
Add Lucas reagent to each test tube.
Shake and observe reactions.
Record observations in the provided table.
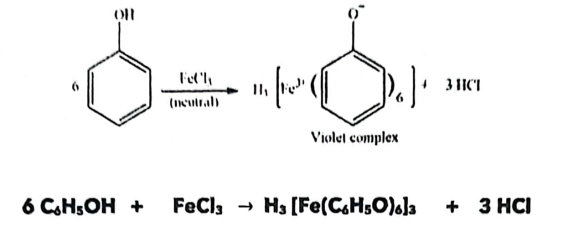
C. Ferric Chloride Color Test (For Phenols)
Principle: Phenols form colored complexes with ferric ions (Fe3+).
Procedure:
Label and prepare test tubes with 3% solutions of phenol, resorcinol, salicylic acid, and ethanol.
Add 1 drop of ferric chloride solution to each.
Observe changes in color and record results.
Questions for Output
Identify substances that produced:
a blue-green solution with K2Cr2O7
an orange solution with K2Cr2O7
a separate layer of the chloride.
Classify the substances as:
primary alcohol
secondary alcohol
tertiary alcohol
phenolic compound.
Determine which test tubes gave different color reactions with FeCl3 and explain any that did not produce color.
Evaluate the necessity of the Lucas test for determining unknowns.
 Knowt
Knowt