Chemistry BASIS 8 : Comp
wow last one!! goodluck on precomps
Unit 1 will not be featured on the PreComp or Comp
Unit 2 : Types & Properties of Matter
Types of Matter — 4-8% (4-8% of the PreComp will be on this)
Matter—something that has mass and volume
mass: amount of matter in an object
volume: amount of space an object takes up
Elements—one type of atom
can exist as atoms or molecules
Molecules—two or more atoms chemically bound together
same or different types of atoms
Compounds—two or more different elements
have a set ratio of elements
Properties of Matter — 4-8%
Elements can’t be broken down using chemistry
Identity determined by # of protons in nucleus
Isotopes determined by # of neutrons in nucleus
Compounds can be broken down using chemistry
Has a fixed chemical composition throughout
Made up of two or more different elements chemically combined
Mixtures contain two or more substances
Homogeneous Mixture—one or more substances dissolved in another substance
Solutions
Solute—the substance being dissolved
Major Component
Solvent—the substance doing the dissolving
Major Component
aqueous (aq) = “dissolved in water”
Heterogeneous Mixture—mixture of substances that remain physically separate
Suspensions—contains large particles that settle out of a mixture
If you can see individual particles, then it’s a suspension
Separation Techniques — 0-4%
Filtration separates solids from liquids and gases
A filter only allows fluid to pass through, leaving solids behind
Filtrate—the fluid that passes through the filter
Cannot be used on solutions (heterogeneous mixtures only)
Distillation separates liquids based on their different boiling points
A mixture of fluids is boiled
Fluid with lowest BP evaporates first—it has the weakest IMFs
Vapor of lower BP fluid cools and condenses into another container
Chromatography separates liquids based on their solubilities
A drop of the mixture goes on a stationary phase
Stationary Phase—stays in place
The mobile phase travels over the stationary phase
Mobile Phase—solvent
States of Matter — 0-4%
Solids have definite shape and volume
Low energy—vibrate in place
Regular particle pattern—touching
Liquids have definite volume and take the shape of their container
Some energy—vibrate and slide past each other
Irregular particle pattern—touching
Gases take the shape and volume of their container
High energy—vibrate, move quickly, bounce off of each other
Irregular particle pattern—not touching, as spread out as possible
Compressible
Changes in Matter
Solid→Liquid—Melting
Liquid→Solid—Freezing
Gas→Liquid—Condensation
Liquid→Gas—Boiling
Solid→Gas—Sublimation
Gas→Solid—Deposition
Temperature, Heat, & Heating Curve — 4-8%
Heat transfers from one substance to another
Exothermic—system releases heat to surroundings
Endothermic—system absorbs heat from surroundings
Temperature—measure of the average kinetic energy of particles in a substance
Heat—energy transferred from one system to another as a result of a difference in temperature (only exists in in the process)
Temperature Conversions
Fahrenheit to Celsius
Cº = 5/9(Fº-32)
Celsius to Fahrenheit
Fº = 9/5(Cº+32)
Celsius to Kelvin
Cº = K-273
Kelvin to Celsius
K = Cº+273
Modes of Transfer
Convection—energy transfer due to the bulk motion of fluids of different temps
Conduction—energy transfer due to the difference in temperature in adjoining regions (transfer through particle collisions)
Radiation—transfer of energy through electromagnetic waves
Heating Curves
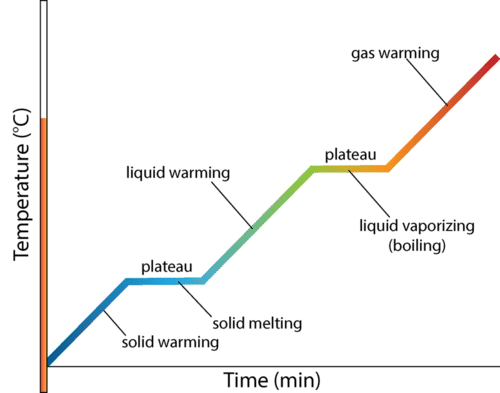
Unit 3 : Periodic Table & Trends
Classification & Families of Elements — 4-8%
Periods go across the periodic table (left & right)
Elements in the same period have the same number of electron shells
# of Shells = Period #
Groups go down the periodic table (up & down)
Elements in the same group have similar properties and same number of valence electrons
# of V.E- = Group #
Characteristics of Metals
Good conductors, lustrous (shiny), malleable, ductile, high melting points, form cations
Alkali Metals
soft, highly reactive, form +1 ions
Alkaline Earth Metals
soft, very reactive, form +2 ions
Transition Metals
most commonly known metals, often have very colorful ions, form ions with a variety of charges
Halogens
diatomic elements, often gaseous at room temp
Noble Gases
inert/unreactive gases, characteristically light up when attached to electricity
Lanthanides & Actinides
reactive with halogens, actinides are radioactive, rare earth metals
Characteristics of Nonmetals
Insulators, dull, brittle, low melting points, form anions
Subatomic Particles & Ions — 4-8%
Isotopes are atoms of the same element with a different number of neutrons
Ions are formed when atoms give up or gain electrons
When electrons are gained, a negative ion is formed
When electrons are lost, a positive ion is formed
Radius, Ionization Energy, Electronegativity — 8-16%
Atomic Radius—the distance from an atom’s nucleus to its outermost electrons
Goes from top right to bottom left
Cs is actually larger than Fr—Cesium is the largest element, not Francium
Ionization Energy—the energy required to remove an electron from a neutral atom in its gaseous state
Electronegativity—an atom’s ability to attract shared electrons in a chemical bond
Polar Bonds—elements have a high difference in electronegativity
Nonpolar Bonds—elements have a low difference in electronegativity
C-H bonds are nonpolar
Same element bonds are nonpolar
Unit 4 : Chemical Bonding
Ionic Bonds — 8-20%
Metal & Nonmetal—electrons are transferred
electrostatic attraction between oppositely charged particles
cation (+), metal or polyatomic ion
anion (-), nonmetal or polyatomic ion
Properties of Ionic Bonds
high melting points
solid does not conduct electricity
both liquid & solution will conduct electricity
some are soluble in water
Ionic bonds must follow the rule of zero charge
Covalent Bonds — 12-28%
Two Nonmetals—electrons are shared
each atom contributes 1 bond
Properties of Covalent Bonds
low melting points
do not conduct electricity
some dissolve in water
Metallic Bonds — 4-8%
Two Metals—electrons are delocalized
atoms are surrounded by a “sea” of shared electrons
Properties of Metallic Compounds
high melting points
do not dissolve in water
conduct electricity as both a liquid & solid
Unit 5 : Molar Mass
Molar Mass — 4-8%
Molar mass is the mass of one mole of a substance, expressed in grams per mole (g/mol).
1 Mole = 6.022 × 10²³ particles (Avogadro’s number)
Molar mass is the sum of the atomic masses of all the atoms in a formula.
Calculating Molar Mass
• For a molecule, add up the molar masses of all elements in the compound.
• Example: Molar mass of H₂O = 2(1.008 g/mol) + 16.00 g/mol = 18.016 g/mol.
Moles & Conversion
1 Mole of a substance = mass (g) ÷ molar mass (g/mol).
Use stoichiometric relationships to convert between moles, mass, and volume (for gases).
Percent Composition — 4-4%
Percent composition is the mass percent of each element in a compound.
Formula
Percent Composition = (Mass of element ÷ Mass of compound) × 100
Example:
• For NaCl, the percent composition of Na is:
(22.99 g/mol ÷ 58.44 g/mol) × 100 ≈ 39.3%.
• The percent composition of Cl is:
(35.45 g/mol ÷ 58.44 g/mol) × 100 ≈ 60.7%.
Empirical Formula — 4-12%
The empirical formula represents the simplest whole-number ratio of elements in a compound.
How to Find the Empirical Formula
1. Convert the mass of each element to moles.
2. Divide each element’s mole value by the smallest number of moles.
3. Round to the nearest whole number if needed.
Example:
• For a compound with 40.0 g C and 6.7 g H:
1. Convert to moles:
• C: 40.0 g ÷ 12.01 g/mol = 3.33 mol
• H: 6.7 g ÷ 1.008 g/mol = 6.64 mol
2. Divide by the smallest mole number (3.33):
• C: 3.33 ÷ 3.33 = 1
• H: 6.64 ÷ 3.33 ≈ 2
Empirical formula: CH₂
Molecular Formula — 4-8%
The molecular formula is the actual number of atoms of each element in a compound. It may be the same as the empirical formula or a multiple of it.
How to Find the Molecular Formula
1. Calculate the empirical formula mass (EFM).
2. Divide the molar mass of the compound by the EFM.
3. Multiply the empirical formula by this factor.
Example:
• If the empirical formula is CH₂ and the molar mass of the compound is 56.08 g/mol,
EFM = 12.01 + 2(1.008) = 14.026 g/mol.
56.08 ÷ 14.026 ≈ 4.
Thus, the molecular formula is C₄H₈.
Unit 6 : Chemical Reactions & Stoichiometry
Acid-Base Reactions
An acid (H- ion) and a base (OH+ ion) react to form a salt and a water.
Precipitation Reactions & Solubility — 4-8%
Precipitation Reaction:
A reaction where two aqueous solutions mix and an insoluble solid (called a precipitate) forms and settles out.
Solubility
Soluble: Substances that dissolve well in water (form aqueous solutions).
Insoluble: Substances that do not dissolve well and form solids (precipitates).
How to Predict a Precipitate
Write the formulas of the reactants and possible products.
Use solubility rules to check if any product is insoluble.
If an insoluble product forms, that’s the precipitate.
Common Solubility Rules
Nitrates (NO₃⁻) and acetates (CH₃COO⁻) are always soluble.
Alkali metals (Group 1) compounds are soluble.
Halides (Cl⁻, Br⁻, I⁻) are soluble except with Ag⁺, Pb²⁺, Hg₂²⁺.
Sulfates (SO₄²⁻) are soluble except with Ba²⁺, Pb²⁺, Ca²⁺, Sr²⁺.
Most carbonates (CO₃²⁻), phosphates (PO₄³⁻), hydroxides (OH⁻) are insoluble except with alkali metals and NH₄⁺.
Redox Reactions — 4-8%
Redox (Oxidation-Reduction) Reactions:
Chemical reactions where electrons are transferred between substances.
Key Terms
Oxidation: Loss of electrons (increase in oxidation state)
Reduction: Gain of electrons (decrease in oxidation state)
Oxidation Numbers (Oxidation States)
An oxidation number is a number assigned to an element in a compound that shows how many electrons it has gained, lost, or shared compared to its neutral atom.
It helps keep track of electron transfer in redox reactions.
Basic Rules for Assigning Oxidation Numbers
The oxidation number of any pure element (like O₂, N₂, or Fe metal) is 0.
For a simple ion, the oxidation number equals the charge of the ion (e.g., Na⁺ is +1, Cl⁻ is -1).
Oxygen usually has an oxidation number of -2 (except in peroxides where it’s -1).
Hydrogen usually has an oxidation number of +1 when bonded to nonmetals, and -1 when bonded to metals.
The sum of oxidation numbers in a neutral compound is 0.
The sum of oxidation numbers in a polyatomic ion equals the ion’s charge.
Combustion Reactions — 4-8%
Combustion Reaction:
A chemical reaction where a substance (usually a hydrocarbon) reacts rapidly with oxygen (O₂) to produce carbon dioxide (CO₂) and water (H₂O), releasing heat and light.
Decomposition Reactions
Rules for Decomposition Reactions
Binary Compounds often break down into their elements:
AB = A + B
2HgO = 2Hg + O2
Metal Carbonates decompose into metal oxides and carbon dioxide:
MCO3 = MO + CO2
CaCO3 = CaO + CO2
Metal Hydroxides decompose into metal oxides and water:
M(OH)n = MO + nH2O
2NaOH = Na2O + H2O
Metal Chlorates decompose into metal chlorides and oxygen gas:
MClO3 = MCl + O2
Limiting Reactant & Yield
Actual Yield = (Percent Yield/100) x Theoretical Yield
Percent Yield = Actual/Theoretical x 100
Theoretical Yield = Limiting Reactant (In Moles) x Moles of Product/Moles of Reactant x Molar Mass of Product
Limiting Reactant: The reactant that is completely used up in a chemical reaction and limits the amount of product formed.
Excess Reactant: The reactant that is not completely used up and remains after the reaction is complete.
Steps to Find the Limiting Reactant
Convert the given mass (or moles) of each reactant to moles of product using stoichiometry.
Compare the moles of product each reactant can produce.
The reactant that produces less product is the limiting reactant.
The other is the excess reactant.
Gas Laws
Gas Laws
Boyle’s Law:
P1V1 = P2V2
Charles’s Law:
V1T1 = V2T2
Gay-Lussac’s Law:
P1T1 = P2T2
Avogadro’s Law:
V1n1 = V2n2
Combined Gas Law:
P1V1/T1 = P2V2/T2
Ideal Gas Law:
PV = nRT
n = number of moles of gas
R = the ideal gas constant, usually 0.0821 L·atm/mol·K
T = temperature in Kelvin