Everyday Chemistry of Life (February 04, 2025)
Water is Life’s Essential Chemical
Water is the most abundant molecule in living organisms.
Water makes up between 60 and 70 percent of total body weight.
Our bodies need water to carry out the basic functions of digestion, excretion, respiration, and circulation.
Without adequate water the body chemical reactions would fail, and our cells would cease to function.
Six Properties of water
Water is liquid at room temperature
The two atoms of hydrogen and one atom of oxygen form polar covalent bonds.
Making water molecules polar
Hydrogen bonds form between the slightly negative oxygen of one water molecule and the slightly positive hydrogen of another water molecules.
The hydrogen bonds between different water molecules continuously break and reform in the liquid water.
Water is able to dissolve many other substances and therefore and therefore is a good solvent.
Hydrophilic substances, such as NaCl(salt) carry a charge and are immediately separated in water.
Hydrophobic substances are not soluble in water.
Hydrophobic substances include large, uncharged particles like fats and oils.
Water is both cohesive and adhesive, allowing it to fill vessels.
These properties allow water to line membranes and provide lubrication.
Blood is 92 percent water, which allows it to stick to the sides of blood vessels and fills them completely.
Providing on excellent transport medium as blood flows through the blood vessels - carrying blood cells and other materials such as gasses and proteins.
Water has a high specific heat.
It takes a lot of energy to raise or lower its temperature.
It takes one calorie of energy to raise the temperature of one gram of water one degree Celsius.
This property helps to stabilize our body temperature as the water in our blood changes very slowly, thus keeping our body temperature relatively constant.
Water has a high heat of vaporization.
This is a measure of the amount of heat needed to vaporize the liquid.
A large amount of heat energy - 540 calories - is needed to convert 1 gram of water to vapor.
Sweating is a means by which heated body water evaporates from the surface of our skins, thus cooling our bodies.
Ice floats
As water cools, the molecules lose energy and move more slowly.
The hydrogen bonds that continuously break and re-form in liquid water stop breaking - and the water turns solid (forming ice)
The bonds hold a specific distance between the molecules.
Making solid water slightly less dense than liquid water.
The trapped air within ice may warm up, thus providing insulation and protection against the cold.
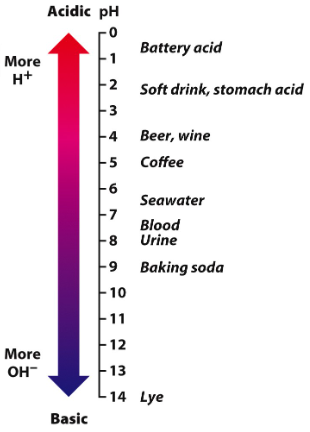
The pH scale measures the concentration of H+
It ranges from 0 to 14.
Lower pH readings indicate a higher H+ concentration and greater acidity (0-7)
Pure water registers 7 on the pH scale.
It has equal numbers of H+ and OH- ions.
A higher pH reading indicates lower H+ concentrations and greater alkalinity (7-14)
The pH scale is logarithmic
Each one unit represents a tenfold change in H+ concentration.
A change from pH 3 to pH 8 would reduce the H+ concentration by a factor of 100,000
Hydrogen ion concentration affects chemical properties.
One of the most important ions is the hydrogen ion, H+
Acidity matters to the human body because it affects the rate of most chemical reaction and the concentration of many chemicals.
The pH of your blood must stay between 7.4 and 7.5 for your cells to function.
If you breathe too slowly, the carbon dioxide level in the blood will rise and the blood can become more acidic
Biological buffers stabilize pH by absorbing or releasing H+
The carbonic-bicarbonate buffering system in blood helps to maintain correct concentration of H+
Organic compounds
Organic compounds contain carbon atoms.
With its four valence electrons a carbon atom can covalently bind with up to four other atoms.
Leading to an almost infinite set of carbon structures.
From simple methane (CH4) to complex ring and chain structures like simple sugars or complex starch molecules.
Attaching functional groups to the carbon structures helps to increase the solubility and reactivity of organic molecules in water - thus making them useful to biological systems.
Four categories of organic compounds are important to living organisms.
Carbohydrates
Lipids
Proteins
Nucleic acids
Carbohydrates
Carbohydrates are the most abundant organic molecules in organisms.
A carbohydrate is composed of carbon, hydrogen, and oxygen atoms.
In a ratio of 1:2:1 - for example, C6H12O6.
Carbohydrates serve as energy source for the human body.
Many carbohydrates are saccharides (sugar)
Monosaccharides
Single sugar molecules - simple carbohydrates
For example. glucose, fructose, ribose, etc…
Disaccharides
Formed form the binding of two monosaccharides.
For example, sucrose (glucose and fructose); lactose (glucose and galactose)
Oligosaccharides and polysaccharides
Longer chains (polymers) of monosaccharides (oligo = few, and poly = many)
Complex carbohydrates.
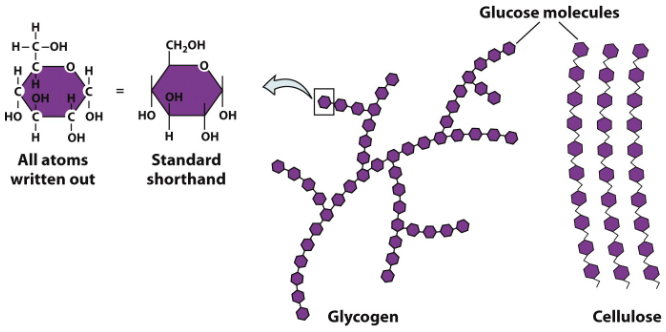
Glycogen is a polysaccharide that is stored in muscle and liver tissue.
It consists of long chains of glucose molecules - stored glucose in animals.
Starch and cellulose are polysaccharides that are found in plants.
They also are long chains of glucose molecules - but are organized differently than glycogen.
Lipids are long-chain organic compounds that are not soluble in water.
Lipids are hydrophobic
Consisting of mostly carbon and hydrogen atoms.
Lipids are fats, oils, sterols, and waxes.
Fatty acids
Energy-storing lipids.
Long chain of hydrogen and carbon atoms with a carboxyl functional group at one end.
The carboxyl group can bind to glycerol molecules to build fats.
Such as triglycerides (with three fatty acids)
And phospholipids (with two fatty acids)
Fatty acids
Unsaturated fats have at least one double bond between adjacent carbons in their fatty acid chains - and bend in shape at each double bond.
Monounsaturated (1 double bond); polyunsaturated (2 or more double bonds).
Unsaturated fat tend to be liquid at room temperature - i.e., oils
Saturated fats have no double bonds between the carbons in their fatty acid chains - and thus are straight in shape.
They are completely saturated with hydrogen atoms and cannot hold anymore.
Saturated fats are solid to semi-solid at room temperature - i.e., butter
A diet high in saturated fats may increase the risk of having a stroke or heart attack.
Phospholipids are both Hydrophilic and Hydrophobic
Consist of a glycerol molecule, a polar head (containing a phosphate group), and 2 non-polar fatty acids.
Their unique structure allows phospholipids to form bilayers when placed in water.
Polar heads face outwards - the non-polar fatty acid tails face inwards.
The cell membrane is one such bilayer.
Steroids are lipids with a common four-ring structure
Steroids and sterols are important to normal growth and development.
Includes cholesterol, sex hormones, and metabolism regulators.
Cholesterol is an integral part of cell membranes.
Allowing for membrane flexibility and growth.
The sex hormones are steroids that are important to the reproductive systems.
Estrogen and testosterone.
Anabolic steroids, which are related to testosterone, stimulate growth of the muscles.
Proteins
All the different proteins found in the human body are formed from just 20 building blocks.
Called amino acids
An amino acid is composed of
A central carbon atom with four groups attached to it.
A hydrogen atom
An amino group (-NH2)
A carboxyl group (-COOH)
A radical group or side chain (R)
The R group determines the activity of the amino acid.
Individual Amino Acids Combine to Form Proteins
Amino acids are gained by peptide bonds.
Peptide bonds form between the amino acids and the carboxyl group of the next amino acid.
The resulting two amino acid compound is called a dipeptide.
As more amino join the growing chain, it becomes a polypeptide.
Polypeptides are linear sequences (polymers) of amino acids.
Polypeptides normally cannot function as proteins.
Must first develop into a unique three-dimensional shape (conformation).
The shape depends on the specific sequence of amino acids.
Protein function emerges from its shape.
That folding and interacting of adjacent amino acids within a polypeptide determine the final shape of a protein (it’s conformation).
The final shape of a protein is either globular or fibrous.
Globular proteins are round and usually water-soluble.
Fibrous proteins are stringy, tough, and usually insoluble in water (provide the framework for supporting cells and tissues).
The shape of a protein molecule determines its function, and the final shape is determined by its primary structure (amino acid sequence).
Changing even one amino acid can alter the folding pattern, with devastating effects on the protein’s function.
In sickle cell anemia, a change in only one amino acid leads to serious consequences.
Enzymes serve as catalysts for biochemical reactions
Catalysts bring the reactants, or substrates, together, so that they can participate in a chemical reaction more quickly.
Enzymes facilitate a specific chemical reaction without being altered during the chemical reactioin.
Unlike the substrates which may be altered during the chemical reaction.
Enzymes rely on shape to function properly.
The active site of the protein is shaped to bind to one specific substrate.
After the substrate binds, the enzyme provides an environment for the specific chemical reaction to occur.
Enzyme function
Nucleic Acids Store and Process an Organisms Hereditary Information.
There are two types of nucleic acids.
Deoxyribonucleic acid (DNA)
Ribonucleic acid (RNA)
DNA exists in the nucleus of our cells.
It contains the hereditary (genetic) information of the cells.
To build proteins
To regulate physiological processes
To maintain homeostasis.
RNA acts as a messenger molecule both inside and outside the nucleus.
RNA serves to regulate cellular metabolism. produce proteins, and govern developmental timing.
Life requires energy
High energy compounds power cellular activity.
Most often energy is available in spurt, rather than as a continuous stream all day long.
Our energy storage system provides short- and long-term storage.
Long term energy storage includes
Glycogen in muscles and liver
Triglycerides packed into specialized storage cells called adipocytes (fat cells)
Short term energy storage uses high-energy system that is reversible and instantly available.
The most common storage system is ATP, or adenosine triphosphate.
ATP powers all cellular activity, forming proteins to contracting muscles.
Adenosine Triphosphate