BIOB10 - Second Half
Table of Contents
Plasma membrane
- Structure
- Function
Mitochondria energy conservation
- Oxidative phosphorylation
Chloroplast energy conservation
Cytoskeleton
- Microtubules
- Actin
Cell Junctions
Studying Cells & Proteins
Plasma Membrane
Structure
Lipid bilayer
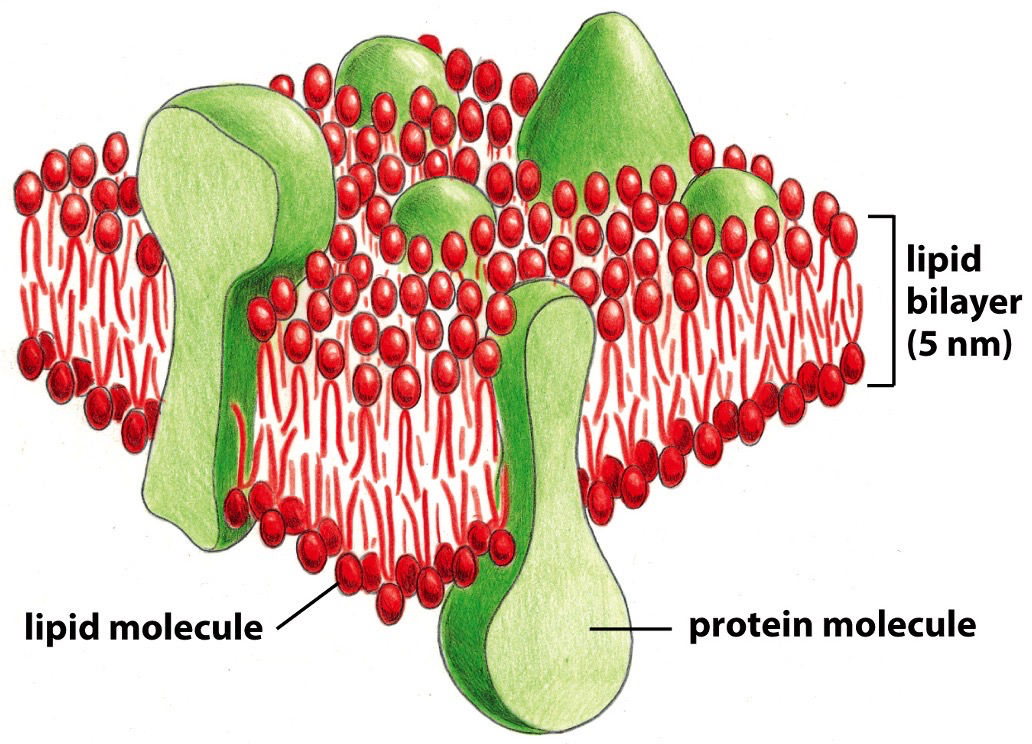
The basic structure, the arrangement comes from lipid characteristics. 50% of the mass of the cell is the bilayer.
- Amphiphilic. Polar head, non-polar tail.
- Cholesterol: Polar head group, rigid steroid ring structure, non-polar hydrocarbon tail.
- Polar covalent bonds: oxygen has higher EN than hydrogen so oxygen pulls electron towards it.
Ideal Structure
- Self healing; small tear in plasma membrane will be okay because the phospholipids react with water and help membrane do sealing.
- Fluidity; phospholipid molecules can easily switch within a monolayer (leaflet), not as easily with ones across from it in the other layer of the bilayer. Moving across is called flip flop, switching in leaflet can be flexion (two tails switch), rotation (whole lipid spins), or lateral diffusion (moves between).
- Depends on the composition. Cholesterol modulates the bilayer. The steroid rings interact with the hydrocarbon chains. Decrease mobility and permeability.
Membrane proteins
Membrane proteins perform a lot of membrane specific tasks, while bilayer maintains shaper. Mitochondria does ATP production however the membrane is almost all protein because of their importance.
Transmembrane proteins: Stuck in the membrane, single pass, multi pass, barrel pore.
Peripheral protein: aren’t physically stuck in the membrane, but are associated with transmembrane proteins.
Association with the bilayer reflects protein function, like how translocators exist in membrane.
Multi pass transmembrane proteins need to pass membrane multiple times, they may originally be separated but to make and specialize into a specific transmembrane protein they will move laterally to get together.
Function
Membrane serves as a barrier, separating organelle content and cytosolic fluid, however a lot needs to cross over. In order to cross over, there are 2 classes of transmembrane proteins: transporters and channels.
Protein-free lipid bilayer where molecules just fuse down concentration gradient.
- Hydrophobic molecules can diffuse right through
- Small uncharged polar molecules, like water, can go partially through.
- Large uncharged polar molecules, like glucose, barely get through.
- Ions, like Na+, cannot get through at all
Transporters
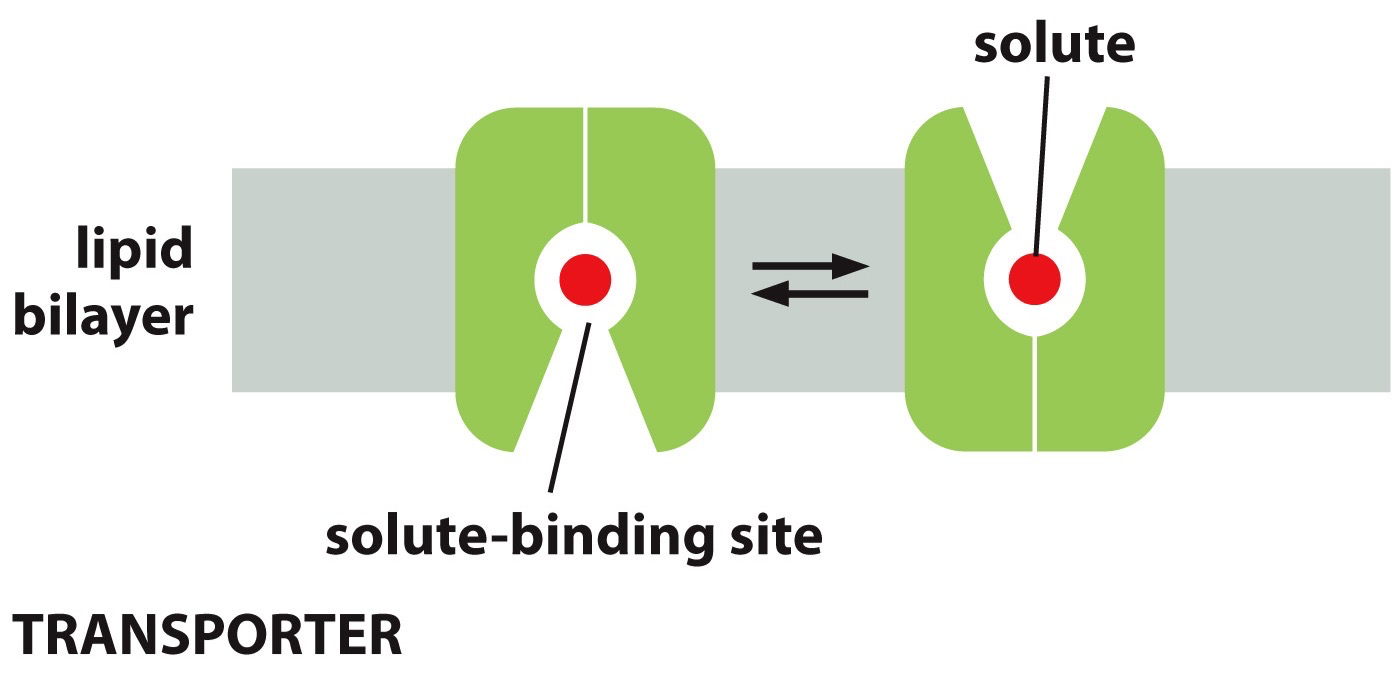
Membrane transport proteins: transfer solutes across the membrane. There is specificity in membrane transport proteins, where they will only do a specific class (like on ion transporter). Multi pass may morph together to form a transporter because of the space between their multiple passes.
Transporters can have a solute-binding site and it undergoes shape change, solute is then successfully transported
Transporters are active or passive, meaning not open or continuously opening.
They are not accessible and then solute binding makes it accessible. Increase concentration, the rate of transport increases till it hits v-max.
Driving active transport is the electrochemical gradient, which means it is moving downhill when it is going with the gradient and uphill if it is against.
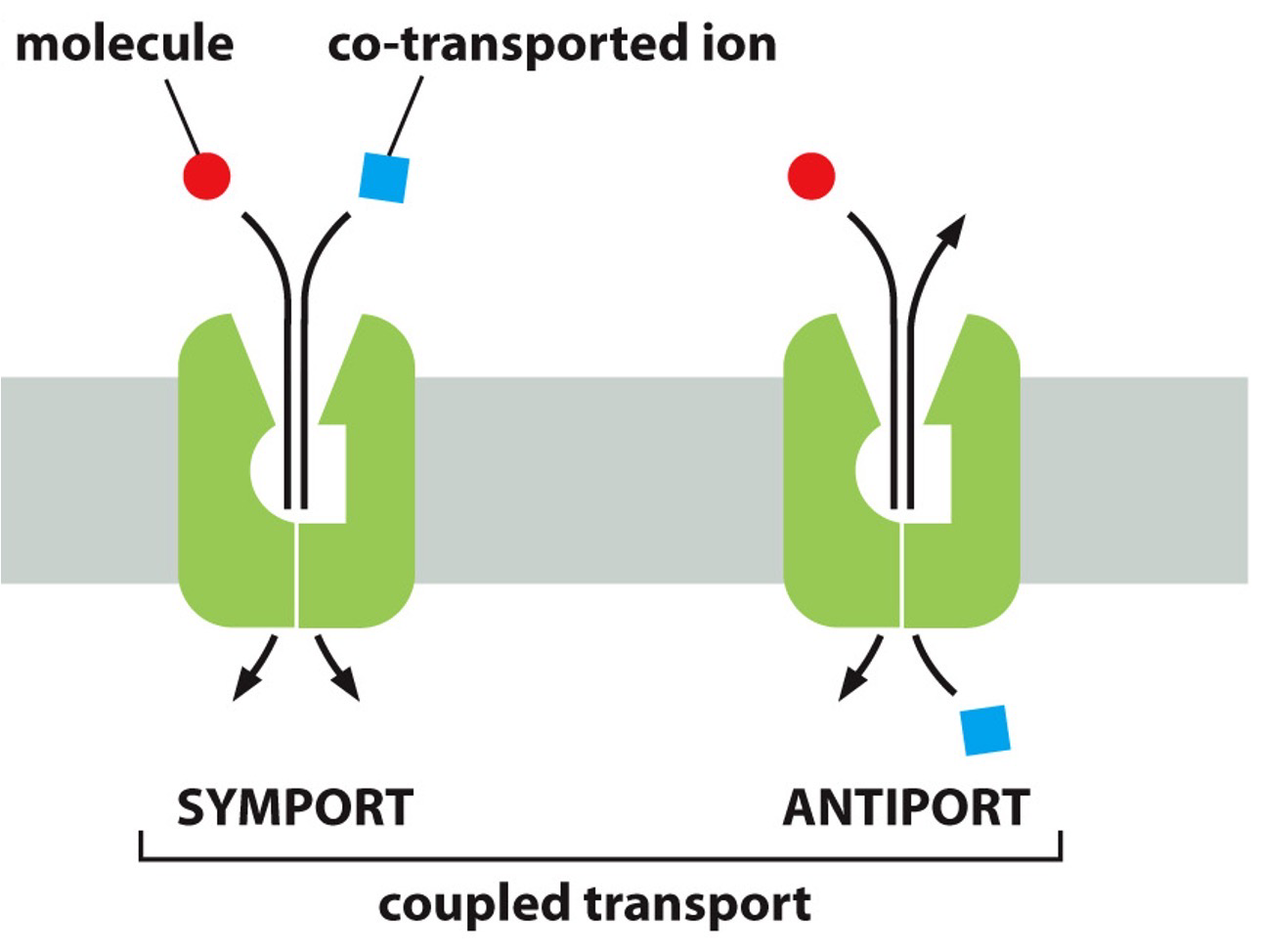
Ion-couple transport - or Secondary Active Transport.
Coupling movement uphill with downhill movement to drive that uphill movement.
Uniporter when one thing goes across, and coupled transporters when things are moving together.
Symporters: Both move in same direction but their gradients are in opposite direction so one is going uphill.
Antiporters: Moving in opposite directions, and their gradients are in same direction so one is going uphill.
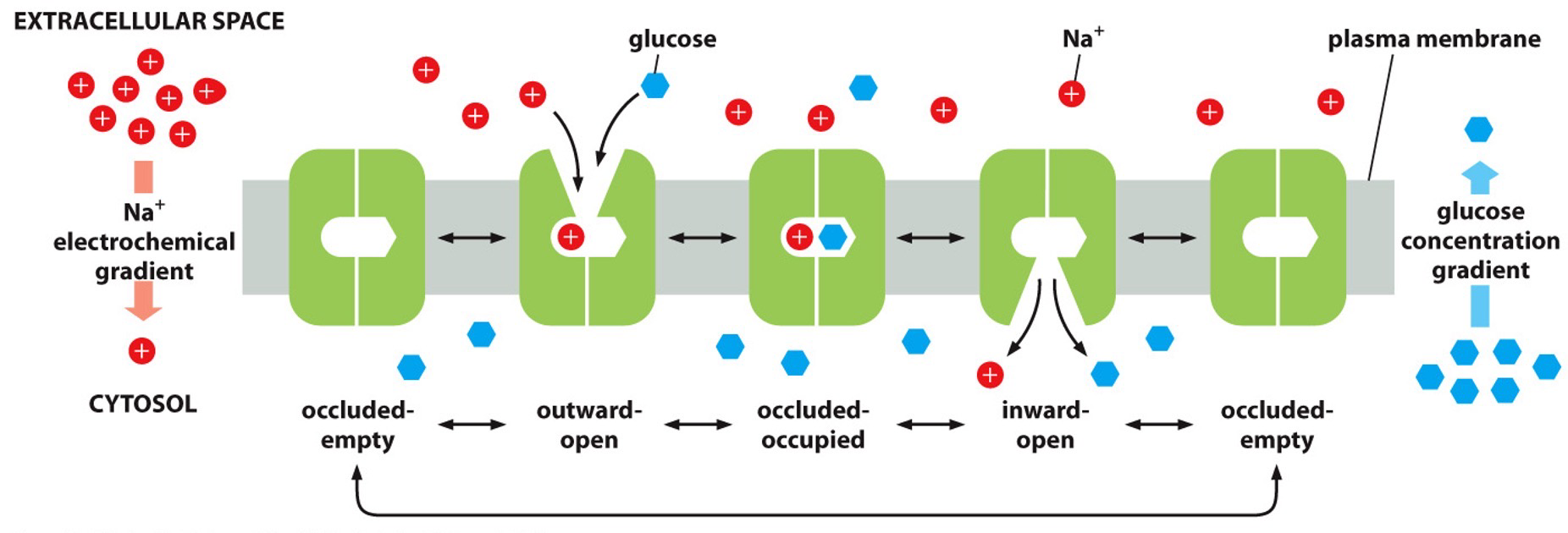
ATP-drive transport - or Primary Active Transport
- ATP-driven pumps
- Hydrolyzes ATP to bring something against concentration gradient.
- P-type pump: hydrolyzes ATP, pumps ions across
- ABC transport: hydrolyzes ATP and pumps small molecules across.
- V-type pump: hydrolyzes ATP and it pumps protons into organelles like lysosomes that need high acidity.
- F-type ATP synthase: works in reverse, it uses proton gradient to drive ATP synthesis.
- Sodium-potassium pump: K+ in, Na+ out, both are moved against electrochemical gradient. Possibly due to ATP hypothesis + they are coupled.
Channels
When open, let continuous flow through, passive.
Form pores across the membrane like aquaporins (water moves through) and ion channels (allows ions directly to move down gradient)
Ion channels
- Not continuously open; they’re gated and when open they’re completely open.
- Selectivity filters what is going to enter and it is situation dependent. Ions have intimate size to pass through. Have shed water molecules to even pass.
- Types of gates
- Voltage-gated: depends on voltage and charge distribution
- Ligand-gated (intracellular or extracellular): ligand changes its shape to open up the gate. Binding causes gated channel to open and allow passing through.
- Mechanically gated: mechanical disruption or mechanical triggering that allows it to stay open or closed.
- Potassium leak channels: permeable mainly to K+, allows potassium to flow along potassium gradient. Helps maintain the membrane potential across the membrane.
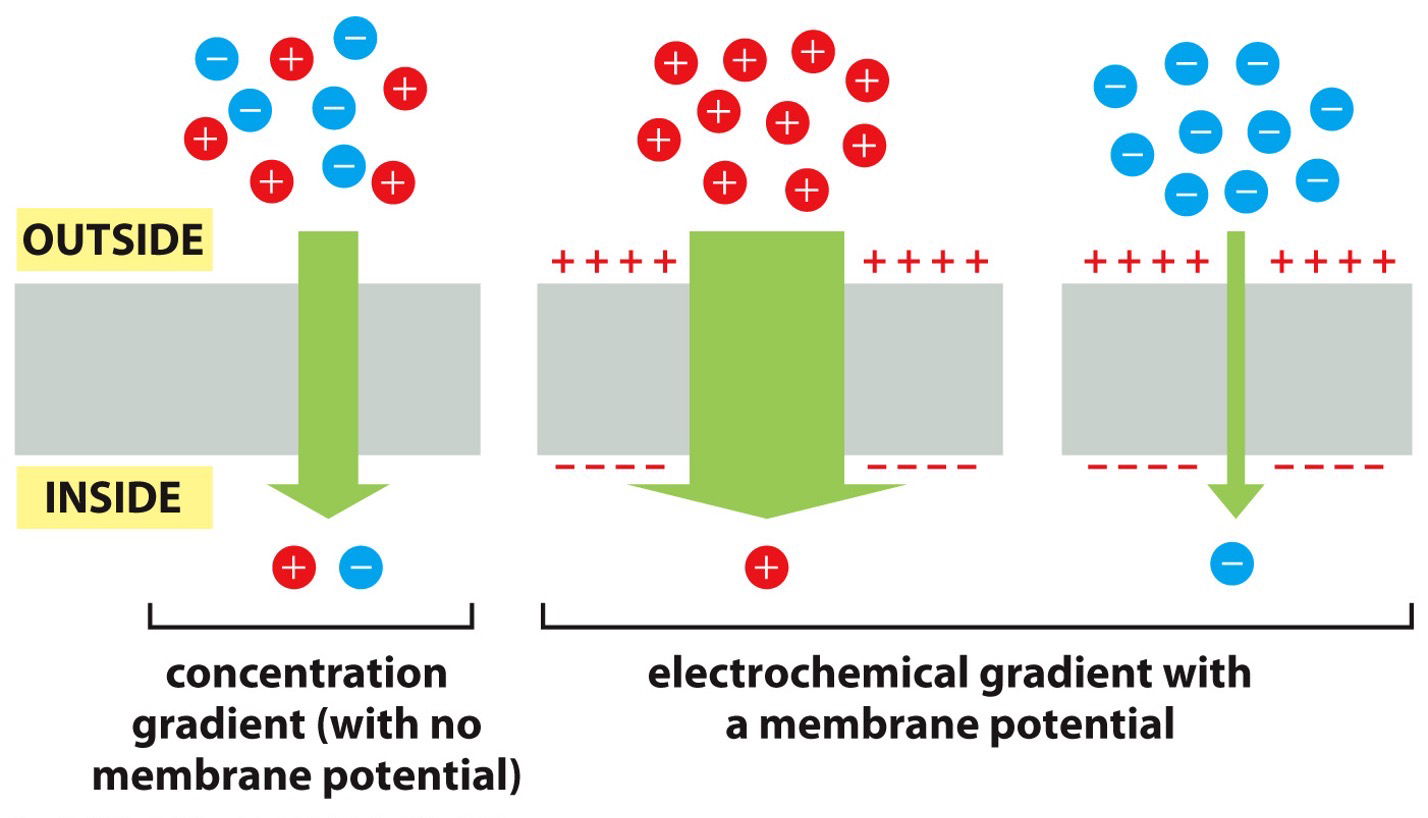
Concentration and Electric Gradients
Concentration gradient
Passive transport: the difference in the concentration on the two sides of the membrane drives the transport and determines its direction. Wants to balance 3:1 → 2:2. Moving down its concentration barrier means to move from more concentrated area to less concentrated area.
- Channel-mediated: down concentration gradient
- Transporter-mediated: down concentration gradient
Simple diffusion: goes right through, probably hydrophobic molecule. Diffuses down concentration gradient.
Electric gradient (membrane potential)
- A difference in charge across a membrane. It has an active potential because inside is negative and positive on outside. Trying to balance by bringing in positively charged solutes inside or opposite.
Both work together
- Concentration asks how many should be brought in, and the electric gradient has a membrane potential saying to bring in more negative or positive.
- Produces net affect
Mitochondria Energy Conservation
Cellular respiration
ATP is produced by energy converting organelles like mitochondria.
Cellular respiration, mainly oxidative phosphorylation, produce ATP. Mitochondria has cristae where ETC and ATP synthesis occurs. Crista junction connects to intramitochondrial membrane.
- Glycolysis
- Pyruvate oxidation
- Citric acid cycle
- Oxidative phosphorylation
Oxidative Phosphorylation
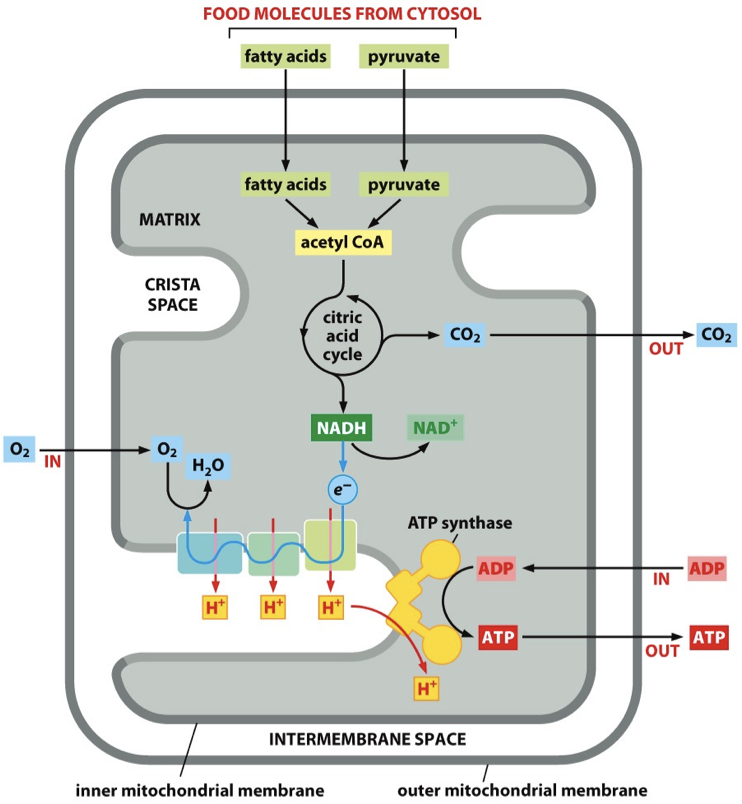
Before: fatty acids and pyruvate are turned into Acetyl CoA by enzymes located in the mitochondria (via citric acid cycle). NADH+ is going to donate electrons to the ETC.
Products: Carbon dioxide and water molecules
Stage 1: Electron Transport Chain (ETC)
Mitochondria ETC = Respiratory chain
ETC protein complexes = Respiratory chain’s protein complexes
Proteins are pumped against the electrons gradient, there are electrons that are passed to protein complexes, generate energy required to pump protons.
Electrons are passed and generate energy that pump protons out.
- Carbon dioxide is produced and released into the environment as a waste product, electrons are lost.
- NADH forms but it doesn’t want to be NADH, so it is converted back to NAD+. Electron is donated from this and fed into protein complex.
Main: NADH passes its electron to first complex in the ETC (becomes NAD+), and the electron is passed along the ETC chain in the inner membrane cristae. Electrons combine with oxygen to make water.
Stage 2: ATP Synthase
Protons have just been pumped and so the protons move back down to ATP synthase, because ATP synthase will take the protons back down the transmembrane electrochemical gradient. The energy from proteins moving down gradient powers synthesis of ATP from ADP and Pi.
Main: Protons move down electrochemical gradient via ATP synthase and this charges ADP + P → ATP
More Information
Respiratory enzyme complexes
- Net result: pumping of H+ out of the matrix across the IMM, driven by energetically favourable flow of electrons
Why protons want to move across gradient
- Protons are kicked out, the protons are positively charged and they wanna balance that out; electric charge difference.
- pH imbalance with the H+, high pH of around 8 in the matrix
- pH gradient + voltage gradient = electrochemical gradient = proton motive force.
NADH & FADH2 are electron donors
Protein complexes have additional members of the ETC that help pass down electrons.
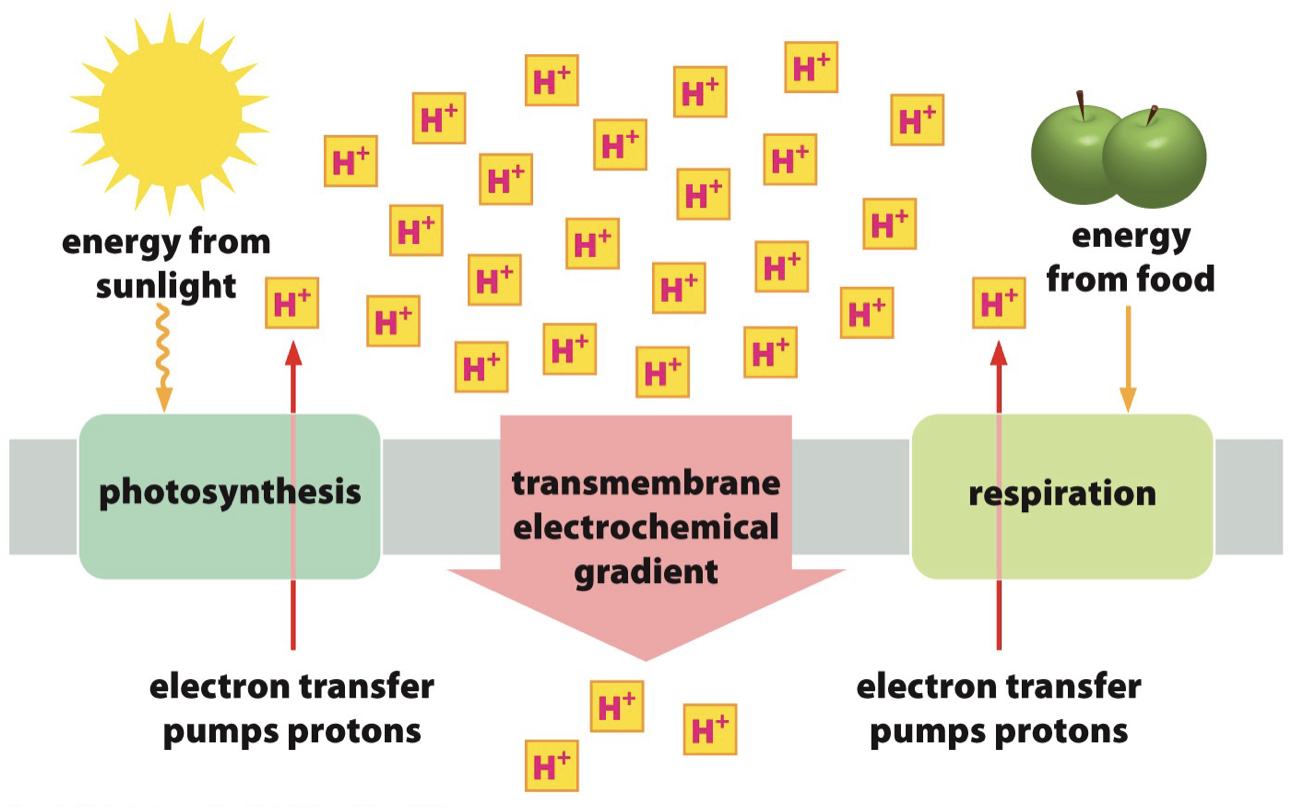
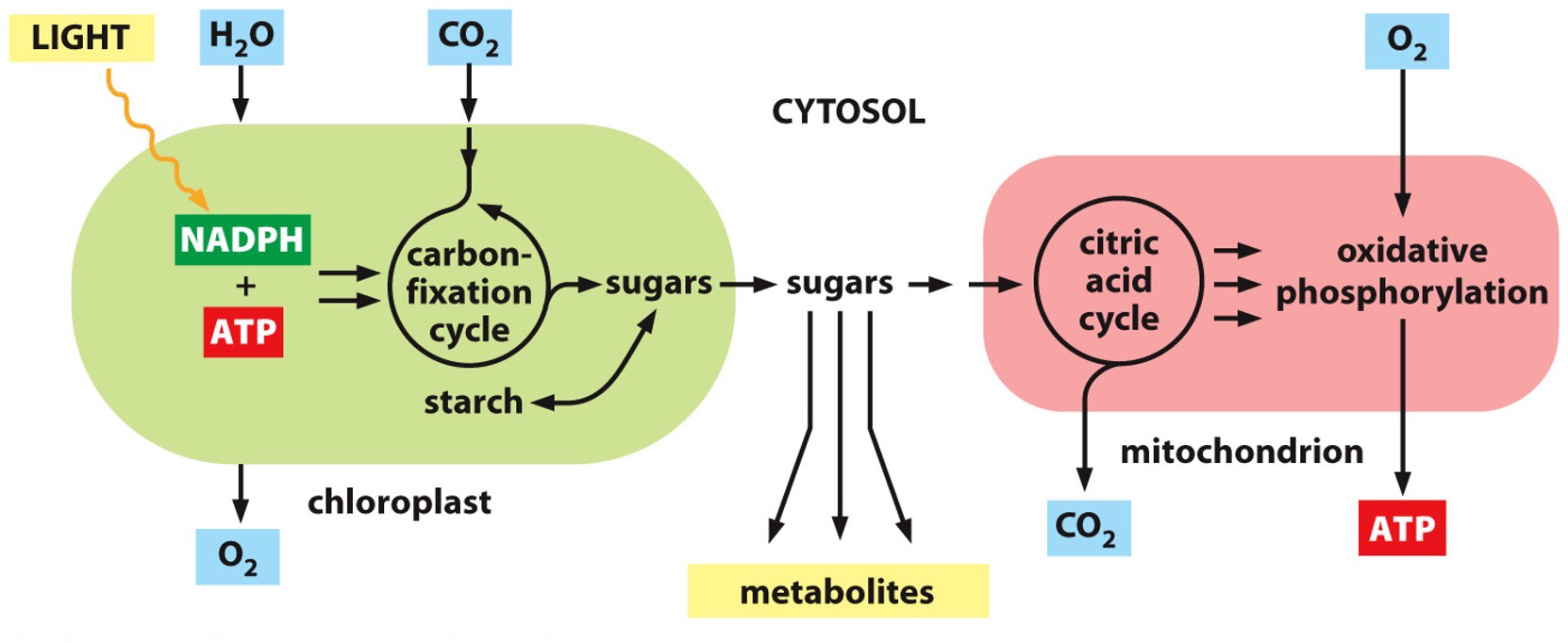
Chloroplasts Energy Conservation
Energy from sunlight is used to transfer protons against the electrochemical gradient.
Chloroplast is similar to the mitochondria → however the thylakoid membrane is the system for ATP production.
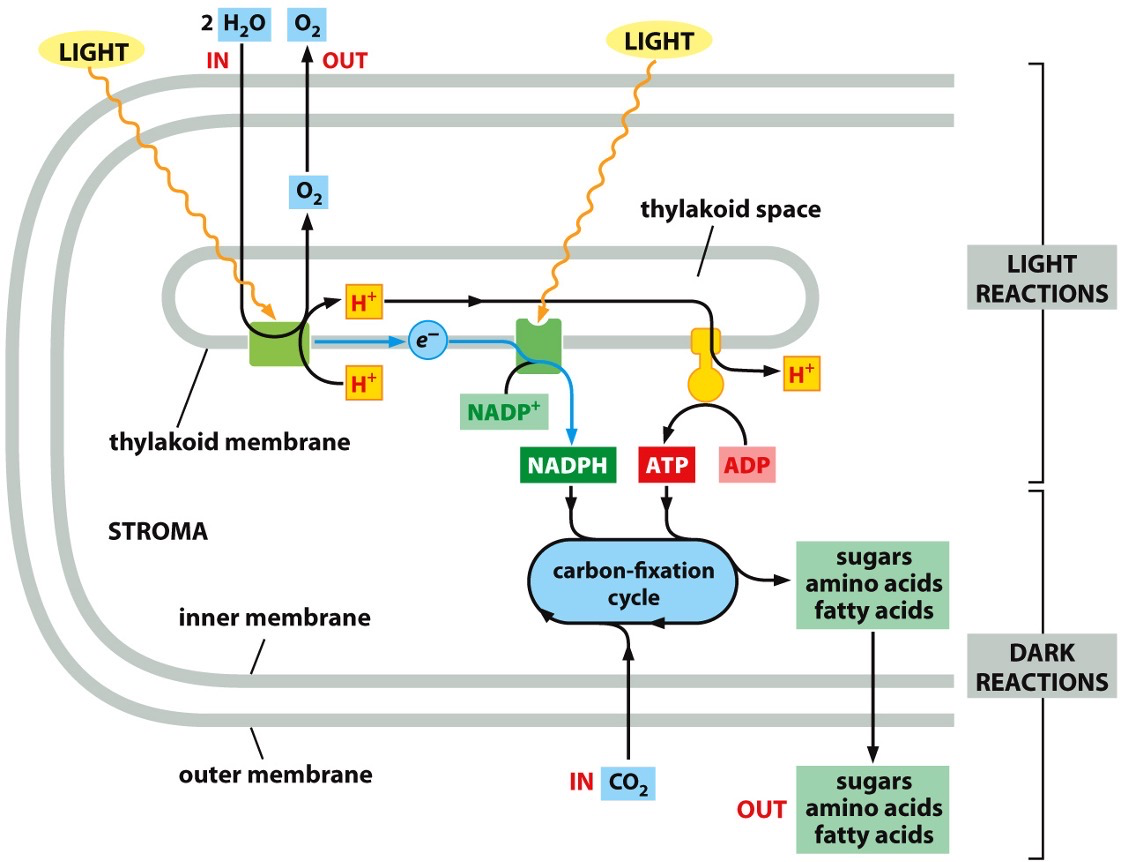
Phosphosynthetic electron transfer reactions (light reactions)
Reaction centre 1 and 2
A photon (quantum of light) knocks an electron out of chlorophyll in the first reaction centre. The electron moves to the second reaction centre. The transferring results in energy that will allow for pumping of protons to happen. The electron gets to the second centre and combines with NADP+ to result in NADPH.
H+ is pumped across the thylakoid membrane (against gradient) and it will move back down electrochemical gradient to produce ATP. ATP and NADPH are the end products, which the dark reactions will use.
More details
- Electrons are regained from water
- O2 gas is produced as a byproduct
- Protons are concentrated in the thylakoid space.
- Creates a large electrochemical gradient across the thylakoid membrane.
Carbon fixation reactions (dark reactions)
NADPH and ATP are fed into the carbon fixation cycle (Calvin cycle). Converts carbon dioxide into sugar, amino acid or fatty acids.
Dark reaction means that it has indirect dependence on light.
NADPH and ATP are converted into stuff that can be transported out and used to make food for the plant.
Cytoskeleton
System in the cell of filaments, that help with moving the cell. Actin filaments border cells. Microtubule filaments are like a highway.
Actin, microtubules, intermediate filaments.
Filament system is dynamic and adaptable. It allows them to do their thing, regulation of dynamic behaviour and assembly of cytoskeleton filaments, eukaryotes to move.
Mitosis
- Sister chromatids line up in centre, spindle apparatus binds and pulls them back. Spindle apparatus is microtubules, breaks down and pull them back. Actin is the contractile ring that pinches in to get two cells.
Crawling cell: Actin can help cells move. Jagged-like edges filled with actin builds up and breaks down at the edges. The cell will chase after this moving bacteria thanks to build up of actin.
Filaments assemble into protein subunits
- Actin filaments: actin subunits (G-actin)
- Microtubules: tubulin subunits
- Intermediate filaments: fibrous subunits that are elongated, no specific name.
- Hydrolysis → makes all these subunits from different filaments with ATP (G-actin) and GTP (tubulin) hydrolysis.
- Filaments use accessory proteins - motor proteins, regulate length, stability, number, geometry of cytoskeletal filaments.
Microtubules
Cell highway system. Extend to cell periphery. Quickly rearrange themselves to form mitotic spindle during cell division. Serve as tracks, help determine position of membrane-bound organelles.
Tubulin subunits have to parts and a bunch of them make up microtubules. - Straight line of them is called protofilament - 13 protofilaments is a microtubule.
- 1 protofilament is thermally unstable and can snap in the middle, however you only want to lose the end.
- Alpha-tubulin: - end, bottom end is the minus end. it’s GTP is not hydrolyzed.
- Beta-tubulin: + end, top end is the plus end. Removal happens at the + end. Beta-tubulin GTP is hydrolyzed, key to how microtubules function.
Rate of addition: Kon (rate of addition of subunits), Koff (rate of removal of subunits.
- Critical concentration [Cc]
- Kon = Koff
Tubulin subunits
- GTP bound - T form subunit
- GDP bound - D form subunit
- Once T is added, a D is taken off by hydrolysis.
- Accumulation of T is a GTP cap. Low GTP hydrolysis rate. If it had a high GTP hydrolysis rate, all would be hydrolyzed and falling off, shrinking the microtubules.
- Can be low, high, or similar rate.
- TIP: T form is growing, D form is shrinking → Dynamic instability
- Catastrophe: Growth → Shrinkage
- Rescue: Shrinkage → Growth
- Dynamic instability
- when they’re been hydrolyzed to D form, the GTP → GDP and subunits want to fall off. This is because they’re not as straight, curved so they come right off, when they’re coming off they’re depolymerizing. Confirmation changes so it struggles staying linear and straight.
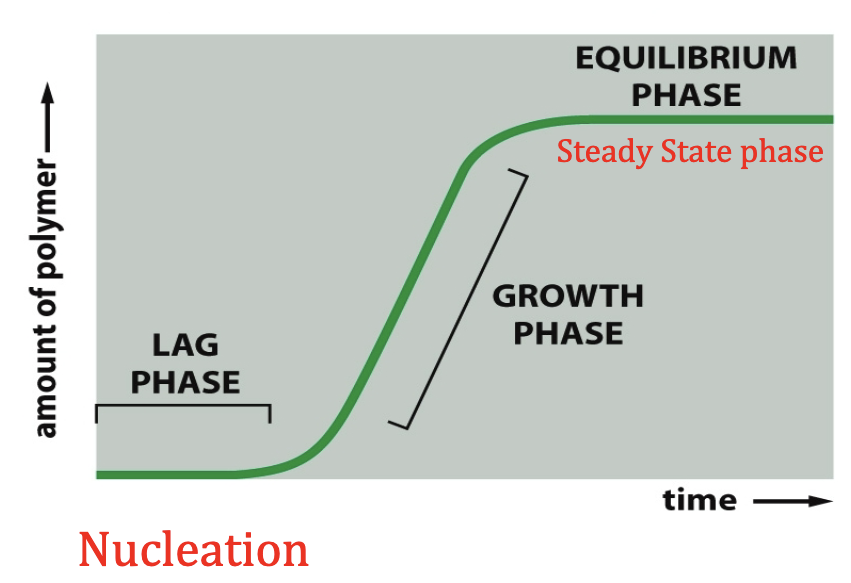
Nucleation
- y-tubulin is involved in the nucleation of microtubule growth.
- Nucleated from the microtubule-organizing centre (MTOC)
- Nucleation depends on the gamma tubulin small complex
- 13 of them, gamma-TuSC
- Gamma-tubulin is like the foundation for the nucleation.
Centrosome
- MTOC for animal cells
- Centrioles are embedded in the centrosome
- All these nucleating sites, plus end extending outward where all the action is happening
Microtubule motor proteins
Kinesins
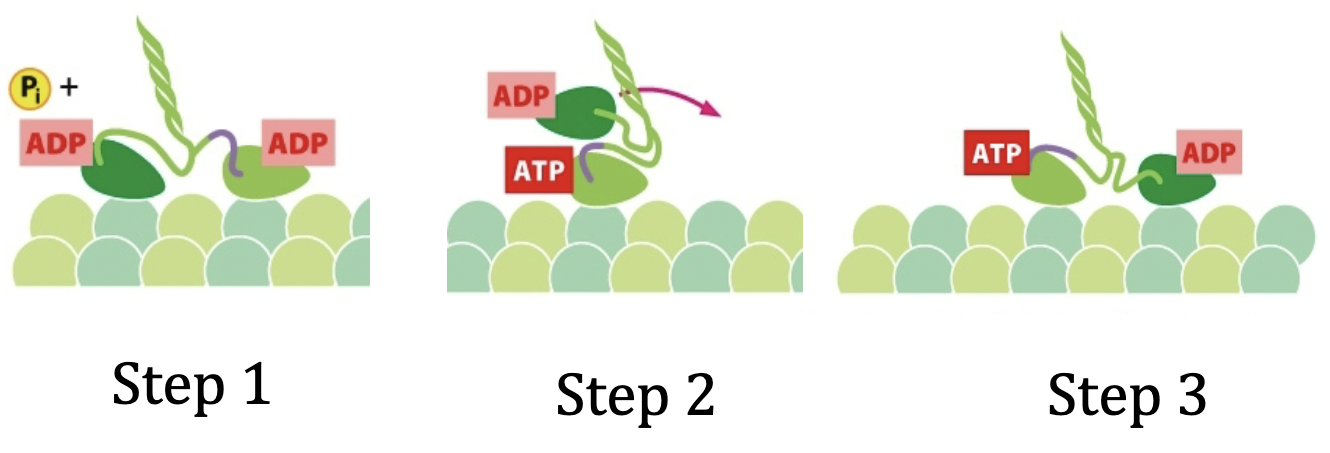
It walks towards the + end of the microtubules. Coiled coil form.
Kinesin step
- Step 0: Lagging head is bound to an ATP, leading head bound to an ADP. The lagging head is behind. Leading strand at front has a neck linker and there is a confirmation change.
- Step 1: ATP is hydrolyzed on lagging strand
- Step 2: Pi from step one leaves and ADP on leading strand leaves. New ATP comes in to attach to leading head, and the ADP bound lagging strand moves forward to become leading head. Neck linker changes confirmation of ATP bound head to allow movement of ADP bound head forward.
- Step 3: The new ADP bound head comes right over in front of the ATP bound strand. There is now a new neck linker in the front on the new ADP bound head.
Dyneins
- Walk toward the minus end.
- Golgi → ER.
Actin
Underlies plasma membrane. Gives it strength, shape, and movement. Muscle contraction.
The actin cytoskeleton is made up of G-actin (globular-actin). Put them all together to get a coiled-coil structure with an ATP in the middle. There is a plus end and a minus end.
Filamentous-actin (F-actin) is the group of G-actin.
Nucleation
- Happening to form initial actin filament. Initial phase is referred to as nucleation process.
- Kon and Koff - rate of addition and removal of subunits with Critical Concentration (Cc).
- You can have a little bit of growth until equilibrium, or you can also have a preformed subunit complex and ignore nucleation phase. It just starts growing.
- Course of polymerization is same as microtubules, however actin filaments have stuff coming on and off of both ends.
Actin can hydrolyze ATP.
- T form and D form. T form comes on and D form comes off.
- kTon, kDoff
- The rates of how fast stuff is added on or off (association/dissociation) can be different rates on either end.
- Minus end and plus end.
- Actin process is called treadmilling.
- Treadmilling: you have a lot of addition happening at the plus end and subunit depolymerization is happening at the minus end. When the rate is equal, the length of the actin is going to stay the same, but it is moving in that end so it is called treadmilling.
- Addition is winning at one end so hydrolysis has to catch up at the other end.
- Can be called steady state treadmilling.
Myosin and actin
- Actin works with motor proteins, specifically myosin. Myosin motor proteins are more like a slide.
- Coiled coil. A bunch of myosins are put together and they’re facing the opposite directions with a bare zone in the middle. Collectively this is called the thick filament.
- A single myosin’s tail is in the tubular structure and the head is poking out of thick filament.
- ATP hydrolysis → conformational change. Movement of Actin.
Myosin process
- Step 1: Myosin head attaches to actin and moves actin back. They’re all holding the actin above their head and passing it back.
- Attached head (1) and next head (2)
- Step 2: ATP comes in and binds to myosin head to detach it from actin.
- Step 3: Cocked, ATP is hydrolyzed (ADP and Pi), and thanks to hydrolysis, this lever arm is facing forward (near 2nd head) instead of backward with 1st head. It tips forward because the lever arm changes position
- Step 4: Phosphate leaves and triggers the power stroke. The head binds to subunit 2 and moves/pulls back into original position but with new subunit attached. This means the subunit with move down and the process will repeat over and over again. The ADP will leave as well once the job is done.
Myosin uses ATP hydrolysis to undergo a conformation change.
- Sliding of myosin along actin filaments causes muscle to contract.
- Voluntary and involuntary
- Involuntary like heart beating or voluntary like picking something up.
- Muscle cell (muscle fibre) consists of myofibrils (the rods) which are made up of sarcomeres (the individual units on the rod)
A sarcomere
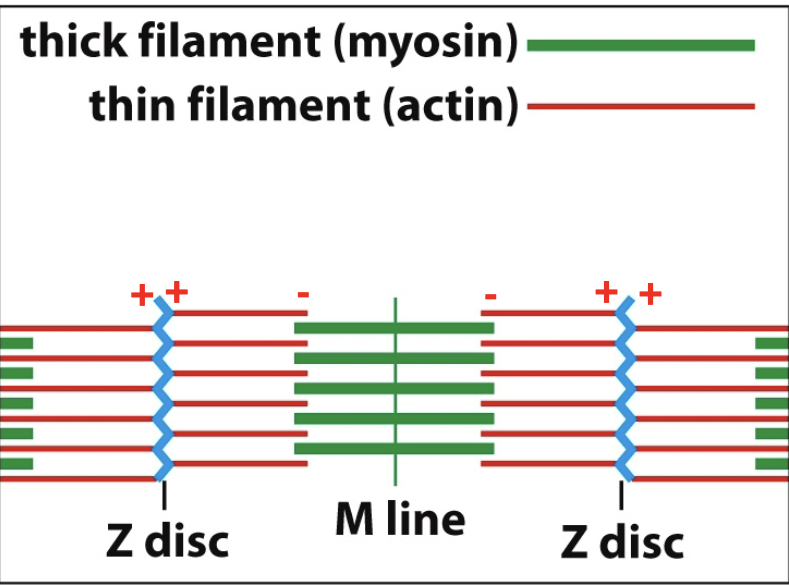
Contracting sarcomere → green moves out and red moves in. Move that pipe back in opposite directions. Myosin heads to right of M-line facing right, Actin that is being held onto is sliding right (vice versa).
Sarcomeres get messages arriving at the plasma membrane, which happens thanks to T-tubules (transverse tubules) and sarcoplasmic reticulum. Sarcoplasmic reticulum is a lace like sheet around the myofibrils that take messages everywhere.
Intermediate filaments
Provides strength and line the inner face of nuclear envelope. Protective cage and allow the formation of tough appendages.
Made up of subunit with N-terminus and C-terminus as a monomer. you then fold a coil dimer with 2 monomers. Then with two of these dimers fold them into a staggered tetramer of 4 monomers. 32 monomers (8 tetratamers) will be the the intermediate filament structure.
Plectin: a family of proteins that link the intermediate filaments to bind to other cytoskeletal components (microtubules are one).
Cell Junctions
Cell interactions govern shape, strength, and arrangement.
Cell-cell junctions
Cells that are bound to each other take on stuff together. Withstand external forces that cells are exposed to.
Linking cells to each other in tissue.
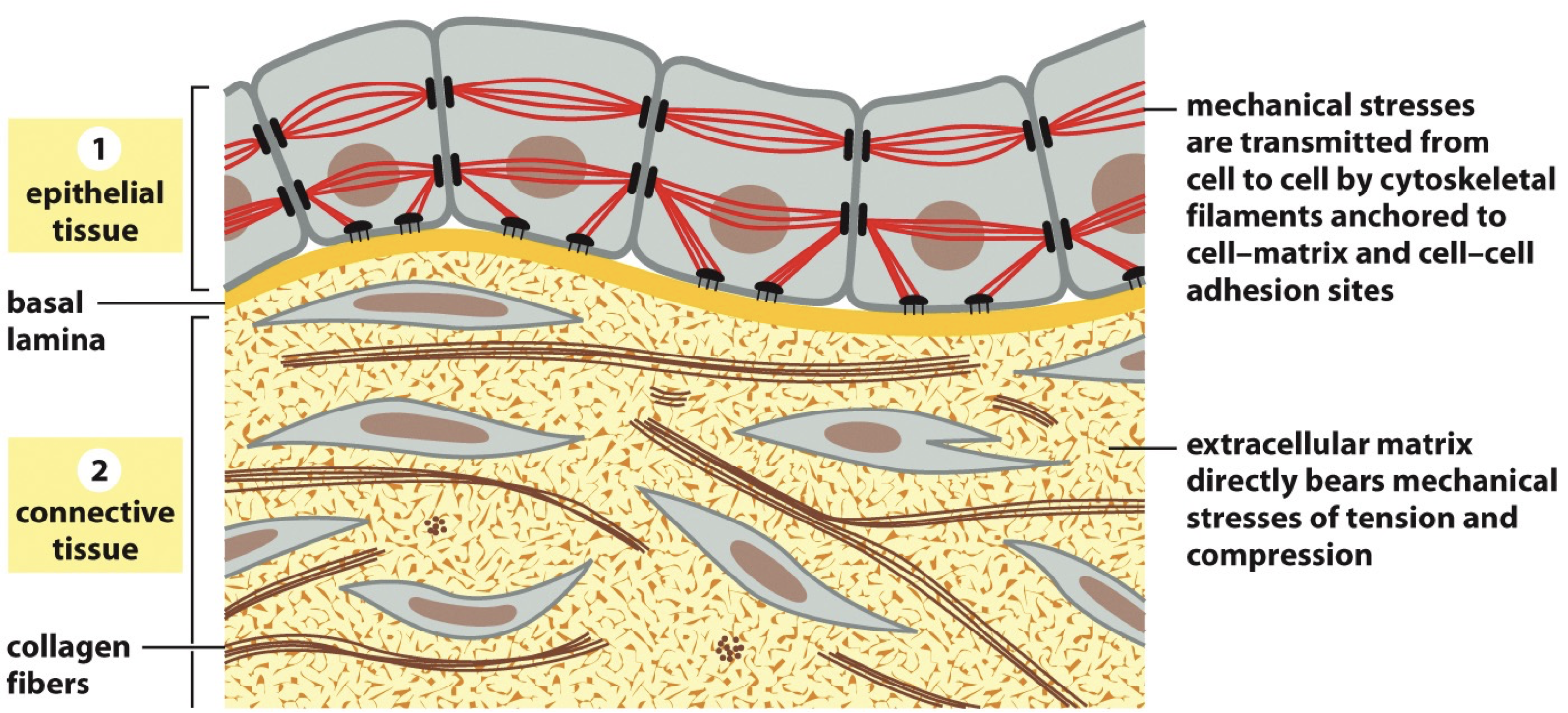 Epithelial tissue: Cytoskeletal filaments in one cell are connected to cytoskeletal filaments in another cell, we refer to this as a cell-cell junction. It is connected to the extracellular matrix through basement membrane/basal lamina.
Epithelial tissue: Cytoskeletal filaments in one cell are connected to cytoskeletal filaments in another cell, we refer to this as a cell-cell junction. It is connected to the extracellular matrix through basement membrane/basal lamina.
Connective tissue: Cells aren’t right next to each other, extracellular matrix is exposed. Cells aren’t really taking on their normal governing cuz they’re not bound to each other. Extracellular matrix is bearing mechanical stresses. Cell-matrix junction.
Anchoring junctions
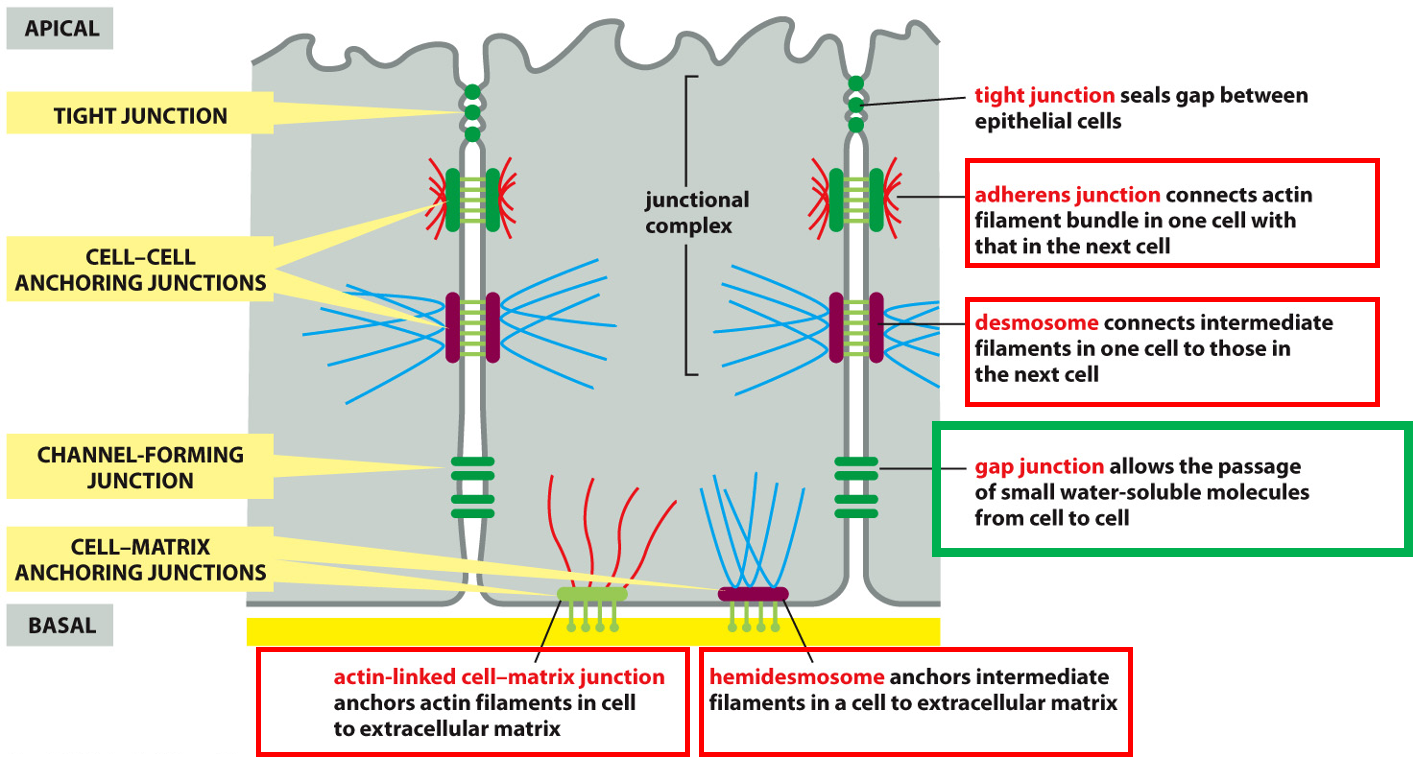
cell-cell:
- Adherens junctions: connects actin filament bundle in one cell with next cell
- Desmosome: connects intermediate filaments in one cell with next cell
cell-matrix:
- Hemidesmosome: anchors intermediate filaments in a cell to extracellular matrix
- Actin-linked cell-matrix junction: anchors actin filament in a cell to extracellular matrix
Junctional complex
- Tight junction: sealing gap between epithelial cells. adherens junction and desmosome.
Gap junction: Small water soluble stuff to pass, channel.
Transmembrane Adhesion Proteins
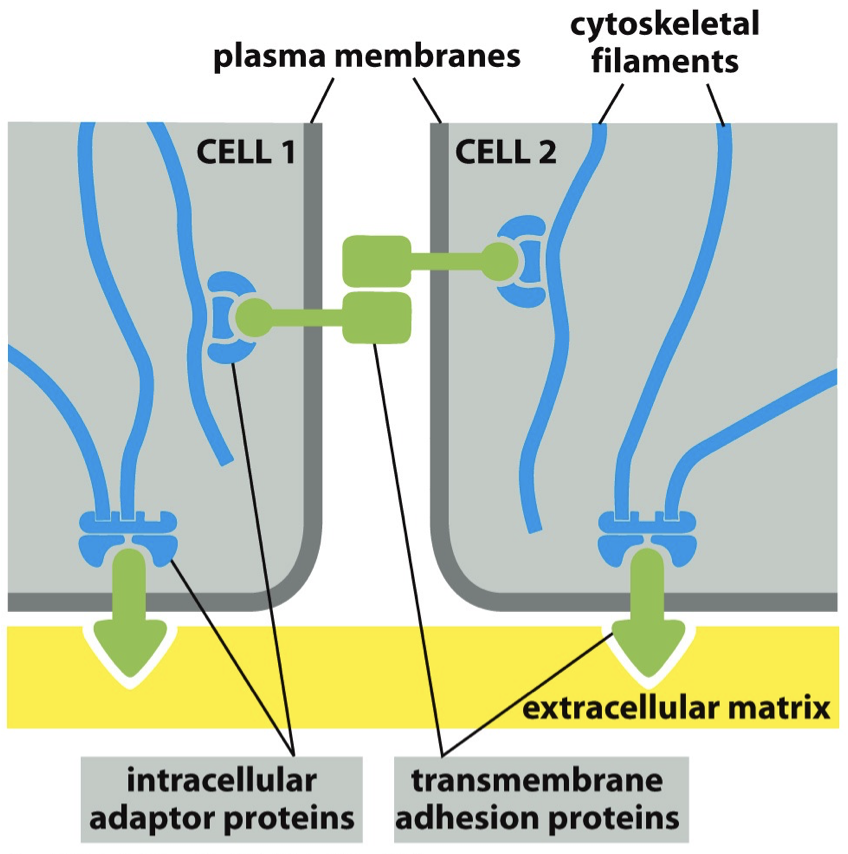
Proteins that allow for binding. Intracellular adaptor protein is gonna help mediate cytoskeletal filament and transmembrane adhesion proteins.
Things stuck in the membrane. It is what binds to matrix and between cells. Stuck in with intracellular adaptor proteins.
2 Superfamilies
- The Cadherin Superfamily
- Mediates cell to cell binding
- Link actin (adherens junction). Link intermediate filaments (desmosomes).
- The Integrin Superfamily
- Mediates cell to matrix binding (actin-linked cell-matrix junction).
- Link actin to matrix. Link intermediate filaments to matrix.
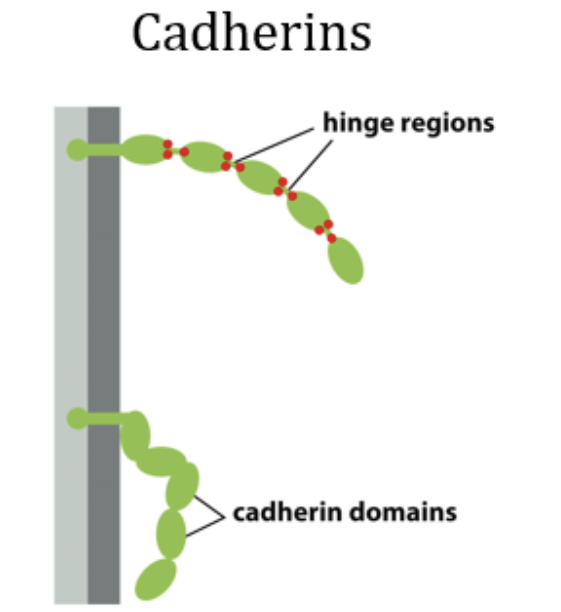
Cadherins superfamily
Dimers
Homophilic if they’re the same. Cadherins are normally homophilic.
Binding happens at N-terminal tips. Fits perfectly with each other in surface-surface binding. Referred to it as extracellular cadherin (EC) domain → part of cadherin is not in the cell.
There are hinges between domains, and those have calcium binding sites. If there is no calcium, cadherin is not willing to bind.
The velcro principle: cadherins typically binds to their partner with low affinity. Applies at cell-cell and cell-matrix willing to bind.
Integrin superfamily
Cell-matrix junction
- Proteins made in cell. Extracellular proteins need to be let out.
- Matrix receptors: tie the matrix outside the cell to its cytoskeleton inside it.
- Epithelial cells: they mediate interaction with basal lamina beneath them.
Integrins
- Principal receptor for cell-matrix interactions
- Has an alpha and a beta. It is heterophilic. It can be inactive and not wanna bind, or active and is ready to bind to each side.
- Strong ligand binding.
- Can be active or switched off. Integrin is not directly bound to actin. Other proteins mediate.
Integrin at hemidesmosome
- Intermediate filament binding to the matrix
- Integrin binding to basal lamina, but it’s not just one thing. Individual parts make up basal lamina.
Matrix Components
Matrix is composed of different proteins and polysaccharides, assemble into mesh work. In close association with the surfaces of cells that produce them.
Parts
Fibrous proteins
- Collagen
- Major protein in extracellular matrix, major component of skin and bones, most abundant (25%) of proteins in mammals. Triple stranded helical structure.
- Alpha chains.
- Type 1 collagen
- Fibrillar collagen → collagen fibrils → collagen fibers. + fibril forming collagen.
- Fibrillar collagen made by cell and secreted into matrix. Help resist tensile force. They have different patterns of tissue based on where they are.
- Collagen type 9 & 12
- Fibril-associated collagens, help organize collagen fibrils. Help by binding onto collagen 1, they will help with formation.
- Elastin
- Helps with elasticity. Allows us to have temporary give.
- Elastic fiber - random coil conformation, they’re still connected, this conformation allows it to do what it does. Cross links.
Proteoglycans (perlecan)
Glycoproteins (Nidogen, Lamina)
Structure and functions of the Basal Lamina
- Helps connect epithelium and connective tissues
- Each cell on either side give structure to basal lamina
- Major components
- Laminin: made up of three chains, hetero-trimer. Has areas devoted to interacting with different areas.
- Type 4 collagen: heterotrimer
Studying Cells and Proteins
Isolating cells
Disrupt what they’re bound to in order to gain access to their components
Step 1: Disrupt the extracellular matrix and cell-cell junctions that hold the cells, mess with the calcium binding agents so they unbind.
Purifying proteins
Proteins perform most cellular processes (catalyzing reactions, nucleotide hydrolysis to do mechanical work, major structural elements of cell)
Sub-cellular fractionation
Find what protein does: isolate it (purify it)
Disrupt membranes to retrieve homogenate.
Ultracentrifuge
- Spinning at high speeds, falling at different moments. Things are extracted.
- Low speed: whole cells, nuclei, cytoskeleton
- Medium speed: mitochondria, lysosome
- High speed: small vesicles, microsomes
- Really high speed: ribosomes, viruses, large macromolecules.
- Centrifuge: good starting step to get initial pellet, need to be purified further
Velocity sedimentation
- Sucrose solution where everything moves down, take a look at what separates.
- Depends on denseness.
Chromatography
Column chromatography: a mixture of proteins in solution is passed through a column containing porous solid matrix
- Charge, hydrophobia, size, affinity for molecules
Ion exchange columns: negatively charged molecules want to interact with positively charged beads. What you get you can assume is negative.
Gel-filtration columns: beads with pores, the molecules that get through that are smaller are delayed and take their time. Larger get out as fast as possible
Affinity columns: beads with certain shapes on the edges, and some proteins will be able to bind to these things.
Hydrophobic columns: if they are hydrophobic, they will go in, if not they will leave.
Epitope tagging
Protein A has corresponding Gene → Gene A, so to find corresponding gene, you add a tag to gene that is transcribed and translated. You undergo regular transcription and translation with the added protein tag. It is known that the protein of interest has this tag. The added tag will want to bind to something specific which will be added, so it is narrowed down.
Narrow it down further: Tandem Affinity Purification Tagging (TAP-tagging)
- Step 1: Purification using affinity column 1, cleave off histidine
- Step 2: protease recognition tag is also on our tag. Take the delayed in beads and protein will snip the protease recognition site, exposing second tag.
- Step 3: Repeat step 1, looking in affinity column 2. Taking multiple steps to narrow down and separate out special protein.
Purified Cell-Free Systems
Isolate the process. Discover what is important. Isolate a process and then look at it in the bigger picture.
SDS Polyacrylamide-Gel Electrophoresis
Step 1: Protein coated with negative charge
- Sodium dodecyl sulfate (SDS) will make everything negative
- Beta-mercaptoethanol - may be what breaks up sulfide-sulfide bonds.
Step 2: An electric field is applied
Small proteins will move faster
Identifying protein function
Identifying what something binds to is necessary to understanding function
Chemical inhibitors: using a chemical to inhibit certain parts of a process to see if they are necessary and what happens without them (cochicine to inhibit microtubules)
Structure: X-ray crystallography and NMR
Amino acid sequence: starts as a gene that is transcribed and then translated, ends up in an amino acid sequence. If the amino acid sequence is similar to another one we understand it may have a similar function.