Acid-Base Balance
Acid-Base Balance Overview
Body’s ability to maintain a steady balance between acids produced during metabolism (ketoacids as last resort for energy, malnutrition, or uncontrolled diabetes and lactic acids for energy during exercise, etc) and bases that neutralize them and excrete them to achieve homeostasis.
Common Health Problems Leading to Imbalance:
Diabetes (leading to metabolic acidosis/DKA)
COPD (resulting in respiratory acidosis)
Kidney disease (which can cause metabolic acidosis and alkalosis).
Learning Objectives
Identify factors maintaining acid-base homeostasis, including various physiological mechanisms and how they interact.
Predict changes in assessment and lab values due to compensatory mechanisms, including how the body compensates for each type of imbalance over time.
Describe the carbonic acid-bicarbonate system, explaining its significance in buffering pH changes in the blood.
Analyze ABG results to differentiate between respiratory and metabolic imbalances by identifying associated trends in gas exchange and acid-base parameters.
Understand causes of acid-base abnormalities, emphasizing predisposing factors, pathophysiology, and the role of organ systems in maintaining equilibrium.
Differentiate assessment findings in patients with various acid-base abnormalities, including clinical signs, symptoms, and the involvement of other organ systems.
Predict treatments for acid-base imbalances based on underlying causes and patient presentations, covering both pharmacologic and non-pharmacologic interventions.
Distinguish between the causes and compensations of acid-base imbalances by understanding the body’s regulatory mechanisms.
Key Terms
ABG (Arterial Blood Gas): A critical diagnostic tool essential for evaluating the acid-base status of a patient, guiding treatment decisions.
Acidosis: A condition characterized by blood pH < 7.35, often resulting from excess hydrogen ions or decreased bicarbonate levels.
Alkalosis: A condition characterized by blood pH > 7.45, typically due to excessive bicarbonate or insufficient carbonic acid.
Buffers: Substances that help stabilize pH in the body by either accepting or donating H+ ions as needed.
HCO3: Bicarbonate (basic/alkalotic), a key buffer in the buffering systems of the body, playing a crucial role in maintaining hemostasis.
PaCO2: Partial pressure of carbon dioxide, which gives insights into respiratory function and metabolic state.
pH: Measure of hydrogen ion concentration in a solution, critical for determining the acid-base status of body fluids.
Acid-Base Balance and pH
Definition: Acids donate hydrogen ions (H+) to a solution, while bases accept hydrogen ions (H+).
Bases do not have their own hydrogen ions so they can take on hydrogen ions from acids to neutralize.
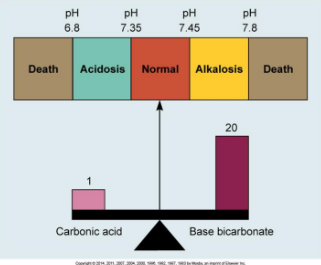
pH Scale: Ranges from 1 (highly acidic) to 14 (highly basic), with a normal blood pH of 7.35 to 7.45 that is critical for normal enzymatic activity and metabolic functions.
pH from 7-8 represents a tenfold decrease in hydrogen ion concentration. Need to neutralize hydrogen ions or excrete them.
pH = Concentration of H+ ions.
Values:
pH < 7.35: Acidosis
pH > 7.45: Alkalosis
Acid-Base Regulation
Metabolic processes produce acids that must be neutralized or excreted.
Body Mechanisms:
Buffers (Cellular/Physiological): Neutralize acids and bases instantly to maintain pH within a narrow range. Fastest-acting (immediate).
Respiratory System: Regulates pH by controlling CO2 levels through changes in the rate and depth of breathing, providing a rapid response to disturbances in acid-base balance. Respond in minutes and takes a few hours to reach maximum effectiveness.
Renal System: Manages bicarbonate levels and excretes excess H+, providing a slower but more prolonged and powerful mechanism for maintaining acid-base homeostasis. Takes 2-3 days they reach maximum effectiveness (only part of body that can maintain long-term).
Ex. Long-term COPD patients are in an acid-base imbalance and kidneys regulate this by reabsorbing more bicarbonate (base) and excreting excess hydrogen ions (H+).
Buffer System
Buffers convert strong acids into weak acids and help prevent significant pH fluctuations in the body. Then, they are excreted by the body.
Types of Buffers:
Cellular Buffers: Primarily involve the exchange of H+ ions with potassium across cell membranes, allowing for quick adjustments in intracellular environments.
Acidosis causes potassium from intracellular to extracellular in exchange for hydrogen ions = hyperkalemia risk. (takes a H+ from blood and kicks out potassium)
When potassium shifts into the blood (extracellular) there’s a higher concentration of potassium in the blood therefore hyperkalemia risk. Too much H+ in blood causes acidosis.
Run CMP to test electrolyte levels.
Alkalosis causes shift of potassium from extracellular to intracellular in exchange for hydrogen ions = hypokalemia risk.
Phosphate Buffers: Function in both intracellular fluid and urine to neutralize strong acids into weak, particularly effective in renal regulation. Breaking these down into weaker acids for kidneys to excrete.
Monohydrogen → one hydrogen. Able to take in another hydrogen.
Ex. In acidosis, monohydrogen phosphate able to take in another hydrogen to help buffer.
Dihydrogen → two hydrogen.
Ex. In alkalosis, dihydrogen phosphate able to give one away to increase pH.
Protein Buffers: Proteins can easily exchange H+ ions, acting as buffers. Attract and release H+ easily.
Amino acids (building blocks of protein) contain both base and acid; they correct abnormalities by either binding to hydrogen ion or releasing a hydrogen ion.
Both phosphates and proteins are breaking these down into weaker acids for kidneys to excrete.
Hemoglobin: In red blood cells, hemoglobin exchanges chloride for bicarbonate (1:1 ratio) as it buffers H+ ions during gas exchange.
Ex. Kicks out a chloride ion and takes in a bicarb, vice versa.
Venous blood is better than arterial blood as a buffer because the hemoglobin isn’t bound to oxygen (deoxygenated).
Carbonic Acid-Bicarbonate Buffer
The key buffering reaction occurs as follows:
HCl + NaHCO3 → NaCl + H2CO3
The carbonic acid then breaks down to CO2 and H2O and can be breathed off.
Dehydration = further breakdown as body holds water and breathes off CO2.
Lungs need to be working for this.
This buffering system plays a vital role in regulating blood pH, especially during respiratory disturbances.
Takes strong acid + strong base and combines them.
Must maintain 20:1 Ratio between bicarb and carbonic acid.
Respiratory System Regulation
Controlled by the medulla oblongata, the respiratory center can adjust breathing rates to regulate acid-base balance through CO2 fluctuations:
Acidosis: Hyperventilation reduces CO2, thereby elevating pH, countering the effects of acidosis. (Kussmal respiration) CONFUSION IS A BIG SIGN.
Caused by excess of carbonic acid → respiratory
Caused by decreased bicarb → metabolic
Alkalosis: Hypoventilation retains CO2 (low and slow RR), subsequently lowering pH to balance alkalosis.
Ex. In a respiratory alkalosis, there’s an decrease in carbonic acid.
In a metabolic alkalosis, there’s an increase in bicarb.
Equation: CO2 + H2O ↔ H2CO3 ↔ H+ + HCO3, reflecting the reversible chemical reactions that occur during gas exchange.
Renal System Regulation
The kidneys play a critical role in long-term acid-base balance through:
Reabsorbing bicarbonate from urine back into the bloodstream.
Excreting excess H+ ions and weak acids to regulate blood pH.
Adjustments made by the kidneys during:
Acidosis (High H+): Excreting H+ ions and retaining bicarbonate (HCO3) to correct the pH imbalance.
Alkalosis (Low H+): Retaining H+ ions and excreting bicarbonate (HCO3) to lower blood pH.
Alterations in Acid-Base Balance
Imbalances arise when compensatory mechanisms fail, classified as:
Respiratory (related to CO2 levels) or metabolic (related to HCO3 levels).
Acidosis or alkalosis, further classified into acute or chronic imbalances, each with distinct approaches for diagnosis and management.
Chronic Imbalances: COPD, asthma, CKD, ESRD(?)
Acute Imbalances: DKA, panic attack, tracheal obstruction
Exceptions: “Acute on chronic” → COPD patient w/ chronic imbalance now has pneumonia and their breathing is messed up. When you look at their ABG, there may be an acute process going on.
COPD patients generally are apart of the “50/50 club”, meaning their CO2 and O2 might both be 50% (need to know baseline).
If a normal person is at 50%, intubate them ASAP.
Clinical Manifestations
Manifestations typically correlate with pH changes rather than the underlying pathological cause:
Acidosis: Symptoms may include CNS depression (e.g., confusion, lethargy, headache, weakness), Kussmaul respirations (deep, labored breathing to breathe off CO2), hyperkalemia (peaked T waves on EKG).
Weakness generally found in acidosis due to denaturing of proteins.
Alkalosis: Symptoms might include CNS irritability (numbness and tingling, restlessness, tetany/muscle twitches → convulsions, seizures, coma), and hypocalcemia (e.g, Chvostek Sign aka facial twitching and Trousseau Sign aka hand & wrist spasm), hypokalemia.
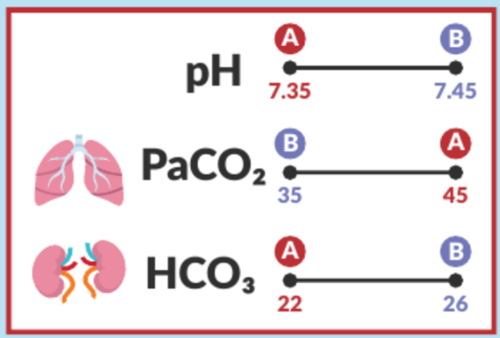
Blood Gas Values (ABGs)
Provides info about acid-base status, body’s ability to regulate pH, underlying cause of imbalance (metabolic or respiratory).
Normal Ranges:
PaO2: 80-100 mmHg
Pa → Partial pressure put on arterial
PaO2 → How oxygen is in the arterial blood
Look at this to determine how much oxygen to give.
PaCO2: 35-45 mmHg
pH: 7.35-7.45
SaO2: 94-100%
Exception: COPD patient
HCO3: 22-26 mEq/L
Respiratory Acidosis “Low and slow RR”
Values:
pH < 7.35 (acidic),
PaCO2 > 45 mmHg (acidic).
Symptoms: Hypoventilation or SOB (retains CO2, slow/shallow respirations), headache, cyanosis (less oxygen), hypotension (vasodilation), hyperkalemia (dysrhythmias), warm and flushed skin, dizziness, CNS depression, confusion, tachycardia, indicating impaired respiratory function.
Causes: Airway obstruction (e.g., asthma, sleep apnea, pulmonary edema, or any kind of impaired gas exchange that blocks air from coming in/getting out), COPD, pneumonia, post-operative, mechanical ventilation, head injury “knocked out”, or CNS depression from drugs (opioid overdose, alcohol intoxication, benzodiazepines), long-term rebreather mask use (CO2 retention).
Management: Find underlying cause. Maintain airway patency, utilize medications (e.g., bronchodilators for pneumonia mainly) to improve airflow, and providing supplemental oxygen as needed (mechanical ventilation/oxygen administration), incentive spirometer.
Compensation: Kidneys excrete H+ (acid) and retain HCO3 (base); HCO3 >26.
If kidneys (HCO3) are within normal range (22-26), there’s no compensation happening.
Metabolic Acidosis
Values:
pH < 7.35 (acidic).
HCO3 < 22 mEq/L (acidic).
Symptoms: CNS depression (lethargy, headache, weakness, hyperkalemia (dysrhythmias), Kussmaul respirations (deep, rapid breathing as compensation), hypotension confusion, N/V.
Causes: Diarrhea (HCO3 in intestines), DKA (not enough insulin to use as energy in your cells = liver breaks down fats = produces ketones in bloodstream often from starvation), renal failure, lactic acidosis (shock aka lack of perfusion/hypotension = produces lactic acid or sepsis), and ingestion of toxic substances (bleach).
Can be caused by a bicarbonate loss or acid gain. Anion gap tells us which one it is. Takes sodium + potassium - chloride - bicarb.
Bicarbonate loss = normal anion gap.
Acid gain = elevated anion gap.
Management: Address underlying causes, possibly including insulin therapy for diabetes, bicarbonate infusion in severe cases, or dialysis for renal failure.
Compensation: Rapid, deep respirations (kussmaul) and LR.
If respiratory (PaCO2) is within normal range (35-45), there’s no compensation happening.
If DKA patient: Give insulin
If kidney patient: Dialysis
Give LR to an acidotic patient because kidneys change LR into a base.
Potassium shifts out of cells into the bloodstream, leading to hyperkalemia.
Acidosis causes hydrogen ions in the blood to be exchanged for potassium from the cells. Excess H+ ions in cells.
Respiratory Alkalosis
Values:
pH > 7.45 (alkalotic/basic),
PaCO2 < 35 mmHg (alkalotic/basic).
Symptoms: Often present with hyperventilation, paresthesia, hypokalemia, (numbness and tingling due to CNS irritability), dizziness/lightheadedness, tachycardia, epigastric pain, nausea, and confusion/lethargy, headache (CNS irritability).
Causes: Often associated with anxiety, high-altitude exposure, pulmonary embolism (PE), hyperventilation (on its own or caused by high fever, bleeds, head injury, ischemic stroke or overuse of mechanical ventilation/head injuries), aspirin overdose (affects medulla oblongata which controls breathing).
Management: Must reestablish CO2 levels may involve strategies like breathing into a paper bag (short-term), and in some cases, administration of sedatives to calm hyperventilating patients (Ativan).
Compensation: Kidneys retain H+ (acid) and excrete bicarbonate (HCO3 < 22).
Metabolic Alkalosis
Values:
pH > 7.45,
HCO3 > 26 mEq/L.
Symptoms: Patients may exhibit restlessness, confusion, and hypokalemia (dysrhythmias), which can lead to muscle cramps and cardiac issues, hypocalcemia (less binding of calcium to make biologically active)
Causes: Severe vomiting, NGT suction, excessive use of diuretics (potassium depletion → body holds onto bicarbonate)/frequent urination, hypokalemia, excessive consumption of antacids (excessive sodium bicarb from rebound acid production).
Management: Encourage CO2 retention by techniques such as breath-holding or bag breathing (short-term), and administering electrolytes if necessary.
Compensation: Hypoventilation → Slow, shallow respirations (holds onto CO2 acid); PaCO2 >45.
Exchange of H+ ions.
Potassium shifts into the cell from the bloodstream, causing hypokalemia.
Caution: Keeping a pt on a rebreather for a long time can lead to CO2 retention → respiratory acidosis.
ALKALOSIS MAINLY AFFECTS CALCIUM