Chapter 5: Reactivity Centers: Functional Groups
Organic Chemistry for Dummies 2nd Edition by Arthur Winter, PhD
Chapter 5: Reactivity Centers: Functional Groups
Functional groups: carbons forming stable bonds to other non-carbon atoms, forming reactive centers.
==Hydrocarbons==
Hydrocarbons: molecules that contain just hydrogen and carbon.
- Includes:
- Alkanes: contain only single bonds and are generally not considered a functional group.
- Inert under most conditions
- Alkenes: molecules containing carbon-carbon double bonds)
- Alkynes: molecules containing carbon-carbon triple bonds
- Aromatics: double-bond-containing ring systems.
Simple hydrocarbons: usually cheap and commercially availble because they’re found as components in crude oil.
@@Double the fun: The alkenes@@
General form of an alkenes:
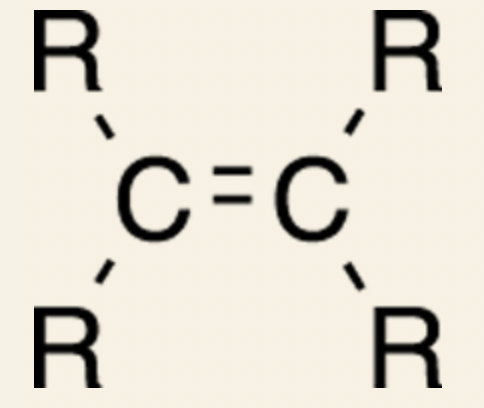
The R is an abbreviation for the rest of the molecule.
- Most often implies a hydrogen atom or a hydrocarbon group
Alkenes: found in natural products (compounds isolated from living organisms).
Alkenes are also used commercially.
- Ethylene polymerizes to make polyetylene (type of plastic used in grocery bags, milk bottles, etc.)
- Polymerize: combines many small units to make large molecules.
Alkenes can also be arrnaged into rings like in cyclopropene (but this is generally not a favorable shape for them to stay in).
They are important to organic chemistry because they are the “go-betweens” in the synthesis of complex molecules.
The names of alkenes generally end with the suffix –ene.
@@Alkynes of fun@@
General form of alkynes:
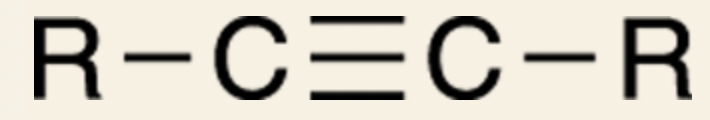
- The simplest alkyne is, acetylene, which has the 2 R groups substituted with hydrogen.
- It is a gas that is used as a welding fuel.
- Alkynes are less common than alkenes in nature, but there are some out there.
- Alkynes prefer to have bond angles of 180 degrees, where the triple bond and the two R groups on either side lie in a straight line.
- Triple bonds do not stretch much, so molecules with triple bonds are generally unstable in rings of fewer than eight carbons.
- Alkyne names end with the suffix –yne.
@@Smelly compounds: The aromatics@@
They are also called ‘arenes’.
Aromatics: consist of rings containing alternating double bonds.
- Example: Benzene (a six-carbon ring with three alternating double bonds).
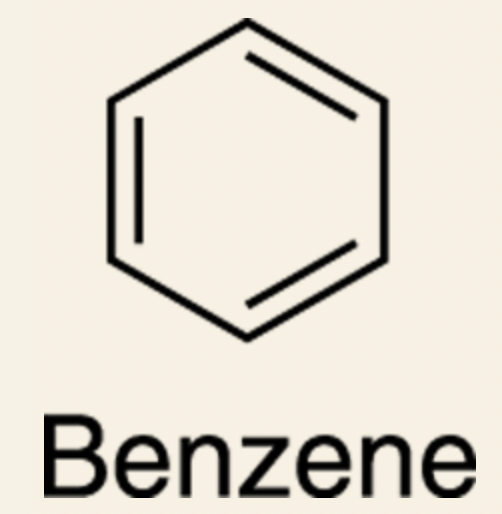
Aromatics have a special property that makes them significantly more stable and less reactive than alkenes.
They are called aromatics because the first of these compounds to be discovered (before their structures were determined) had funky smells.
Many compounds made by living organisms contain aromatic rings.
==Singly Bonded Heteroatoms==
- Heteroatoms: atoms other than carbon or hydrogen.
- Include such important atoms as the halogens, oxygen, and sulfur.
- These are the components of the halide, alcohol, ether, and thiol functional groups.
@@Happy halides@@
Halides are organic compounds that contain one or more halogens.
4 halogens that you frequently see in organic compounds: fluorine, chlorine, bromine, and iodine.
General form of halides:
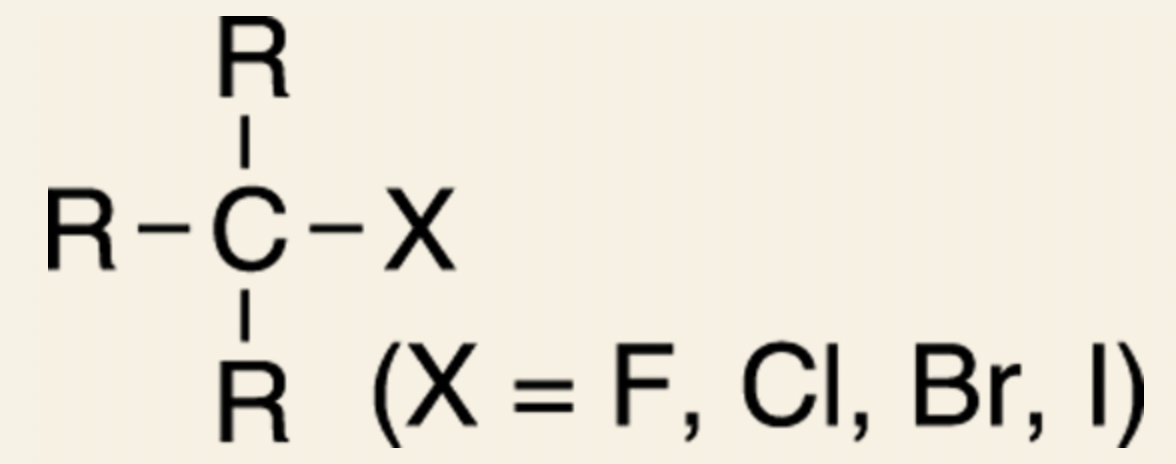
Halides are not common in the natural world., and when they are found, they’re often in compounds that are toxins.
- Commercially, halides are used as propellants in aerosol cans such as hairspray and spray paint, solvents such as chloroform, and as refrigerants.
- Also as insecticides such as DDT. This is banned in the United States because it has caused unwanted harmful side effects in wildlife and in humans.
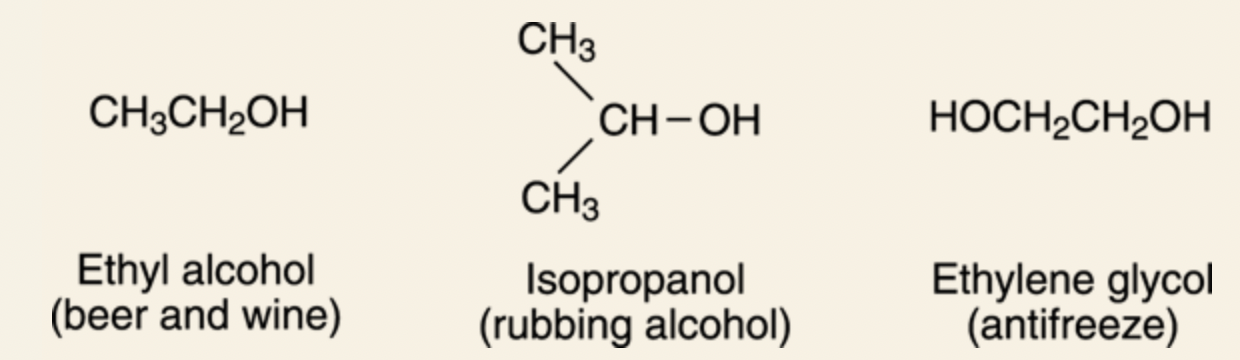
@@For rubbing and drinking: Alcohols@@
Alcohols consist of the general formula R-OH.
The names end with the suffix -ol.
Some examples include: ethanol (alcohol found in beer, wine, and liquor), isopropanol (rubbing alcohol), and ethlene glycol (used in antifreeze).
Alcohols are also found in natural products.
- Like, sugar contains many -OH groups.
@@What stinks? Thiols@@
- They are foul-smelling compounds and contain the formula R-SH.
- Thiols: sulfur analog of alcohols/in other hideously unpleasant compounds.
- Like: flatulence, garlic, sewage, skunk spray, etc.
- \