GCSE Biology Revision "Required Practical 6: Photosynthesis"
Required Practical Setup
Materials Needed
Boiling tube
LED light source (preferred due to low heat output)
Normal light bulb (requires additional setup with a beaker of water)
Sodium hydrogen carbonate solution (provides carbon dioxide)
Pond weed
Experimental Procedure
Positioning the Light Source
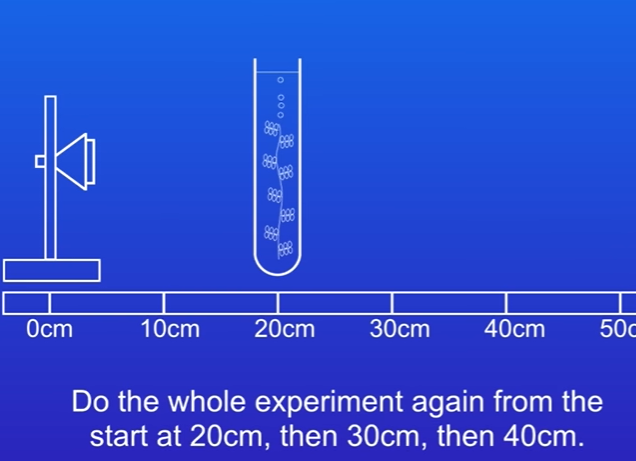
Place the boiling tube 10 cm away from the LED light.
If using a normal light bulb, insert a beaker of water between the light and boiling tube to absorb excess heat produced by bulb.
An LED light is used as these don't release very much heat; too much heat would change the temperature of the experiment.
Preparing the Boiling Tube
Fill the boiling tube with sodium hydrogen carbonate solution — Sodium hydrogen carbonate solution releases carbon dioxide, which is needed for photosynthesis
Place a piece of pond weed into boiling tube with the cut end facing upwards.
Allow the setup to acclimatize to the conditions of the boiling tube for five minutes.
Observing Oxygen Production
Observe for gas bubbles produced from the pond weed; this gas is oxygen generated by photosynthesis.
Start a stopwatch and count the number of bubbles produced in one minute.
Repeat this for two more trials and calculate the mean no. of bubbles per minute.
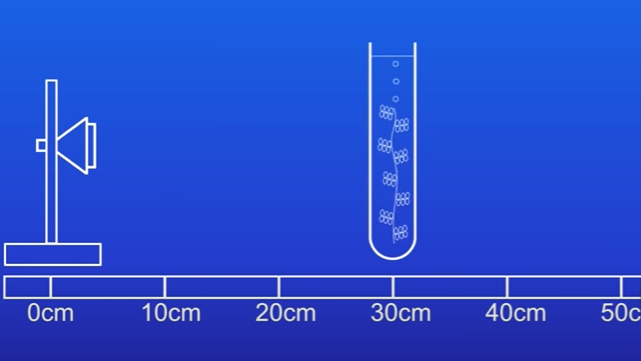
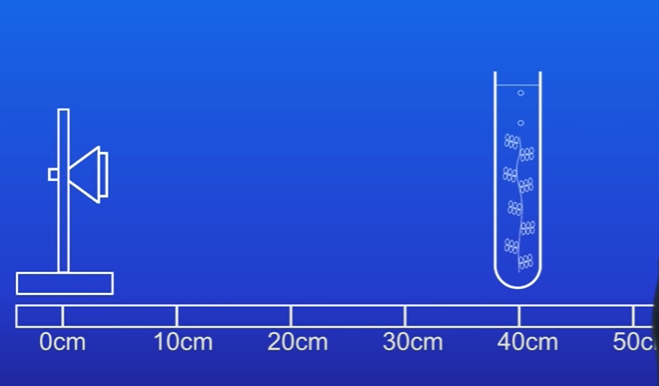
Changing Distances
Repeat the experiment at distances of 20 cm, 30 cm, and 40 cm:
Challenges and Solutions
Problems Identifie
Bubble Counting Difficulty: no. of Bubbles can be produced too rapidly to count accurately.
Bubble Size Variation: Bubbles are not always the same size: for example, a large bubble would count the same as a small bubble.
Bubbles vary in size, making consistent counting challenging.
Solutions
Measure the volume of oxygen produced instead of counting bubbles. Do this by: Placing pond weed under funnel and catching the bubbles in a measuring cylinder filled with water. Then use measuring cylinder to measure the volume of the oxygen gas produced
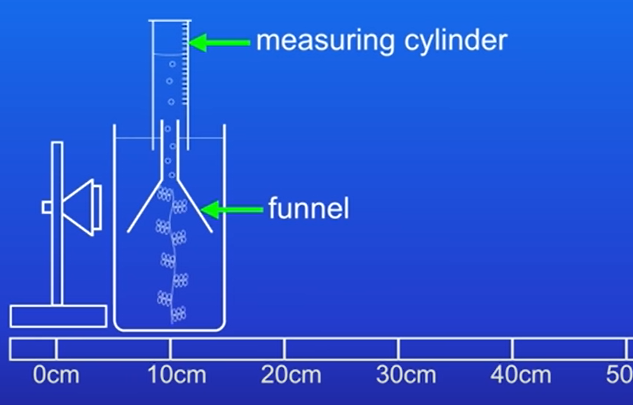
Collect bubbles in a measuring cylinder filled with water for accurate volume measurement.
Data Interpretation for Higher-Tier Students
Graphing Results
Plot the mean number of bubbles per minute or the volume of oxygen per minute against the distance from the light source.
If we plot the mean number of bubbles per minute/volume of oxygen per minute against the distance from the lamp to the pond weed, then we get this graph:
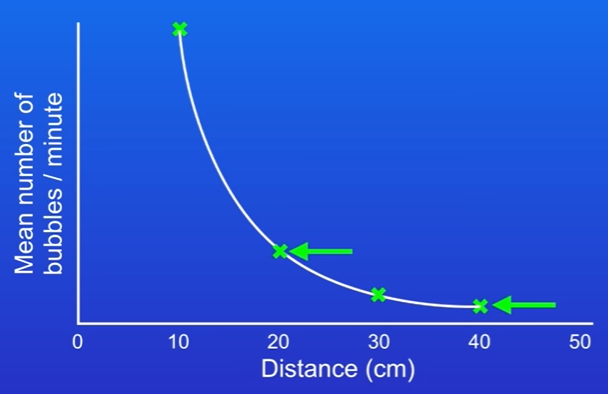
Inverse Square Law
Key Observation: if we double the distance, then the number of bubbles per minute falls by a factor of four (this is called inverse square law) (y=k/x, light intensity= 1/distance²)
Example: Moving from 10 cm to 20 cm reduces bubbles produced from the pond weed by four times.
Again, moving from 20 cm to 40 cm also results in a fourfold decrease.
Explanation: This phenomenon occurs because light intensity decreases with the square of the distance, thus affecting the rate of photosynthesis.
The reason for this is that if we double the distance, the light intensity falls by four times(x1/4), and because we need light for photosynthesis, that causes the number of oxygen bubbles to fall by four times.
