pH homeostasis/ Respiratory Role/Blood gas analysis
LEARNING OBJECTIVES
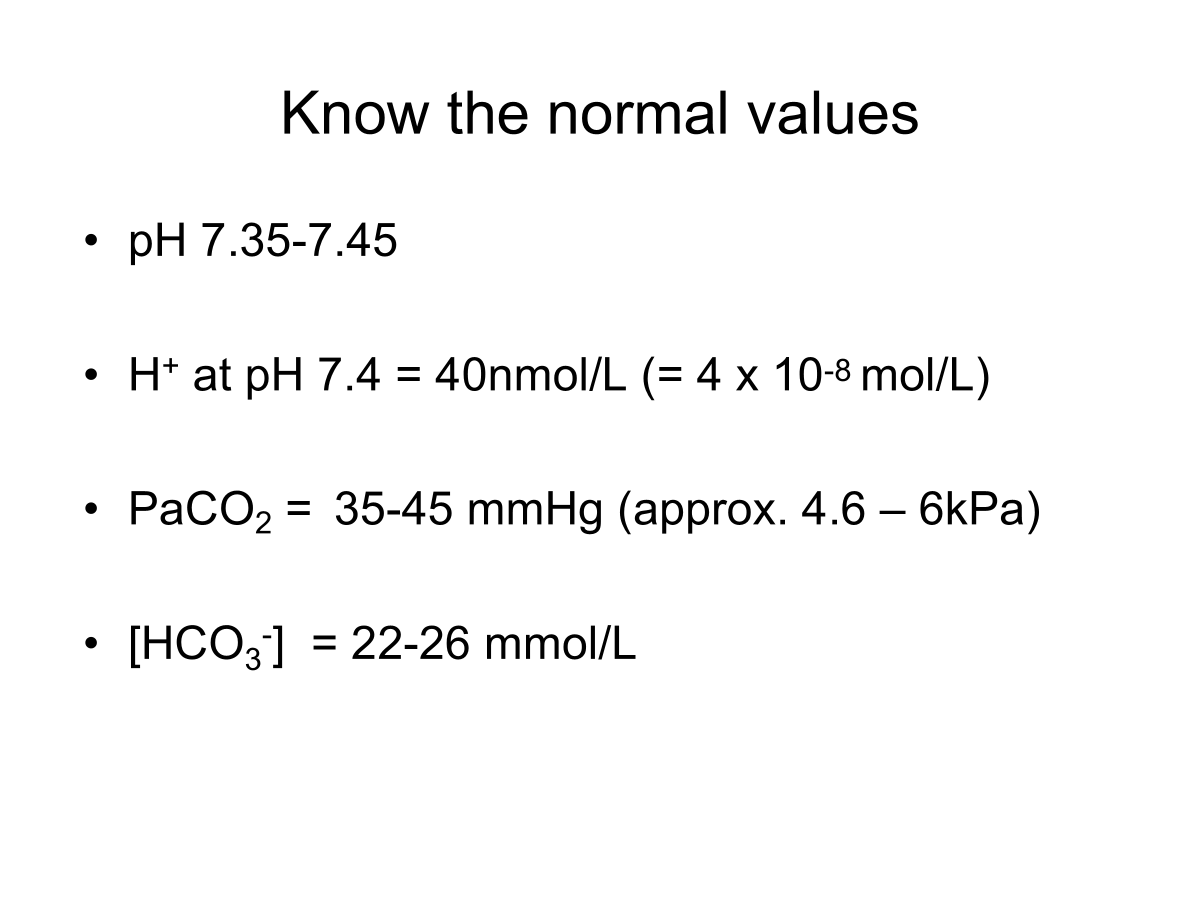
State the normal plasma pH range.
Describe factors involved in pH homeostasis.
Appreciate the rationale for the relationship between blood plasma pH, [H+], PaCO2 and [HCO3-] and the relevance of the respiratory and renal systems to the carbonic acid-bicarbonate bugger system and pH control.
Explain, with examples, why respiratory acid/base disturbances can arise and the compensation and corrective mechanism.
Interpret basic arterial blood gas analysis.
Acid-Base Balance/Imbalance
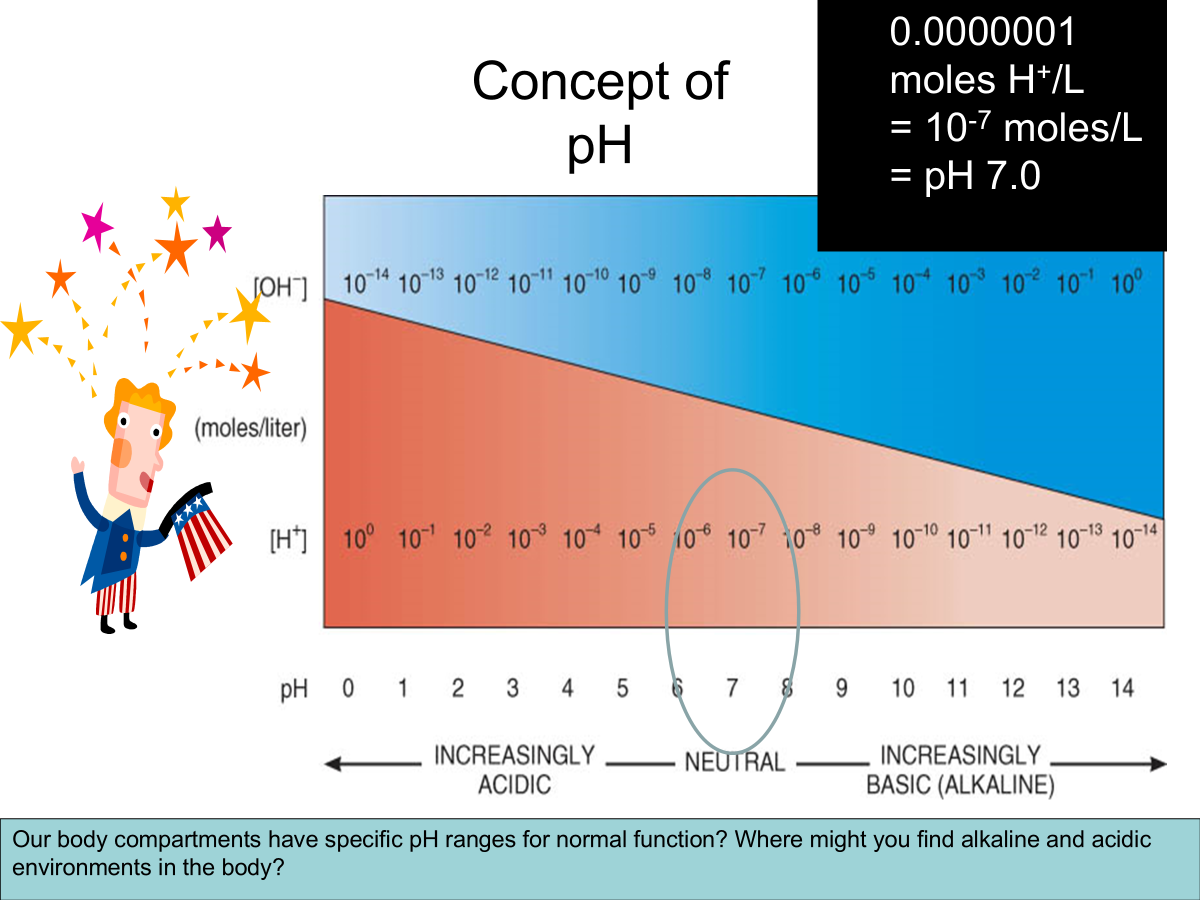
pH → A solutions acidity or alkalinity.
The power of hydrogen.
Negative log10 of [H+] in moles/L
pH = -log10[H+]
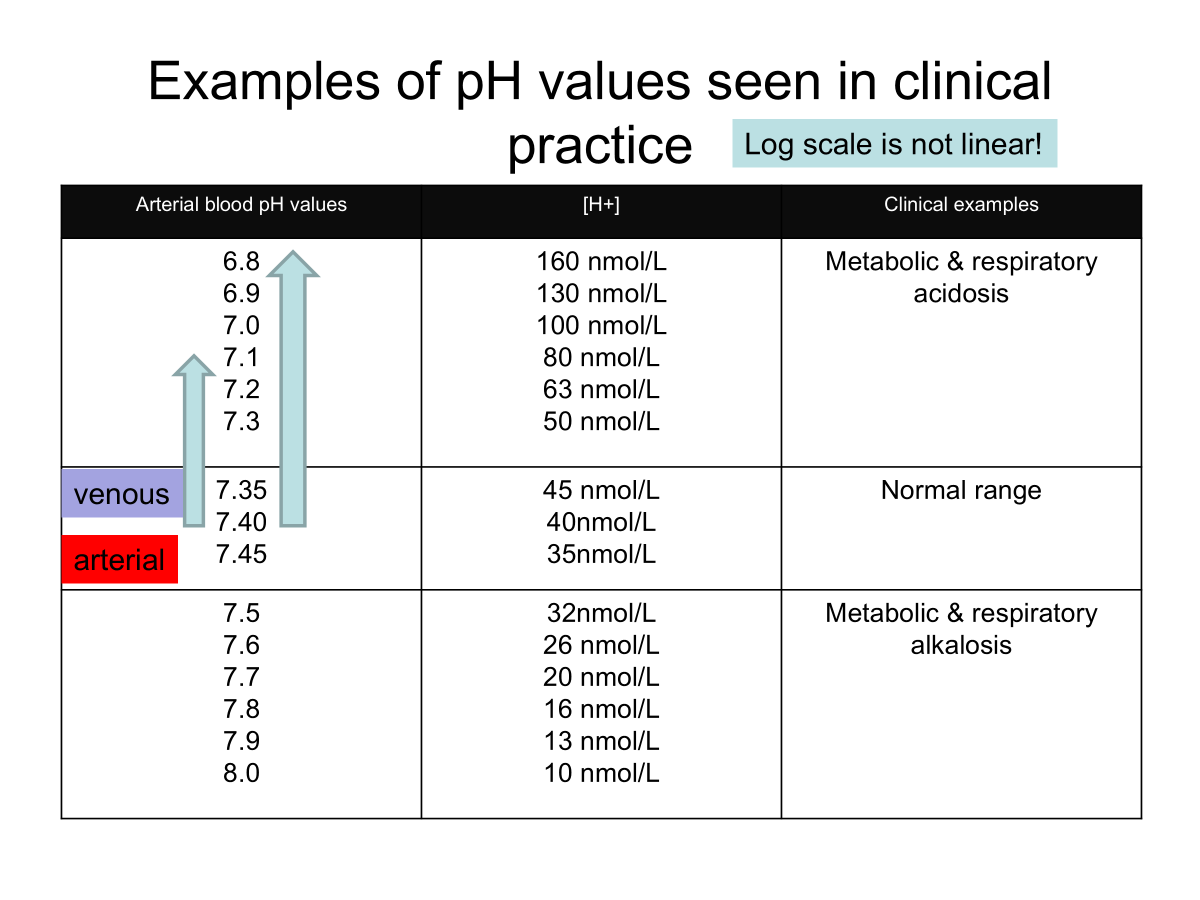
Normal pH range of systemic arterial blood - 7.35-7.45
7/4 = 40nM/L H+
Acidosis → Blood pH below 7.35
Depresses the CNS through depression of synaptic transmission (coma).
Alkalosis → Blood pH above 7.45
Overexcitability of the PNS then CNS through facilitation of synaptic transmission (spasms, convulsion and death)
Metabolic production of acid produces free H+
HA ←→ H+ + A-
Sources of acid:
Carbonic acid
CO2 + H2O ←→ H2CO3 ←→ H+ + HCO3-
Non volatile acid produced from nutrient breakdown e.g protein metabolism.
organic caids from intermediate metabolism e.g lactic acid.
Lines of defense against pH disorders
Chemical buffers
Adjusting ventilation to change PaCO2
Adjusting renal acid or alkalis excretion
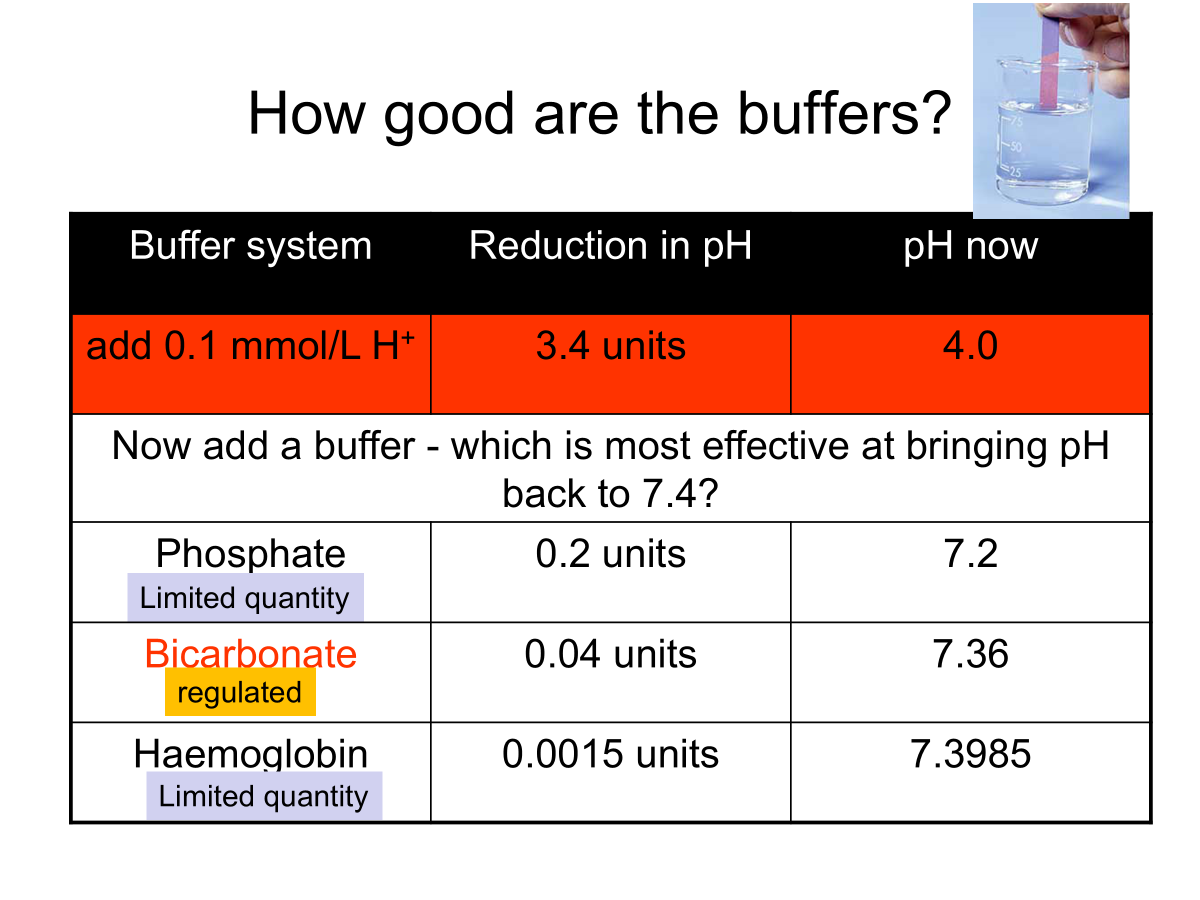
Buffers
Why? Prevent rapid, drastic changes in pH.
Binds free H+
Most effective around the pKa, the pH at which the acid is 50% ionised.
3 main extracellular buffer systems:
Protein buffer system - Hb and plasma proteins
Phosphate buffer system e.g in bone
Carbonic acid-bicarbonate buffer system
Carbonic acid-bicarbonate buffer system
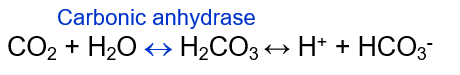
At a pH of 7.4, bicarbonate ion conc is about 20 times that of carbonic acid.
pK for the reaction is 6.1.
This system can not protect against pH changes due to respiratory problems in which there is too much or too little CO2.
CO2 is regulated by the Lungs
detected by Chemoreceptors
Seconds
HCO3- and H+ regulated by the Kidneys
H+ excreted
HCO3- reabsorbed or excreted
Hours/days
Henderson-Hasselbach Equation
Chemical equation that describes the derivation of pH as a measure of acidity in biological and chemical systems.
Need to know the relationship between pH, PaCO2 and HCO3-
Applied to the bicarbonate buffer system
Bicarbonate is the major buffer system in blood and ECF.
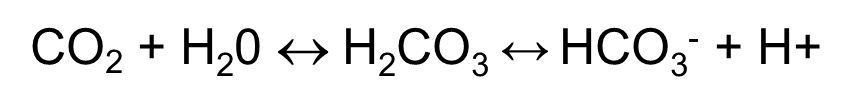
pH = pK + log[base]/[acid] = pK + log HCO3-/H2CO3
At pH 7.4, most H2CO3 exists as dissolved CO2.
pH = 6.1 + log HCO3-/CO3
6.1 = pKa for the system.
Quantify CO2:
PCO2 x its solubility coefficient (0.03mmol/L/mmHg)
i.e CO2 = 0.03 x PCO2
pH = pK + logHCO3-/CO2
= pK + logHCO3-/0.03 x PCO2

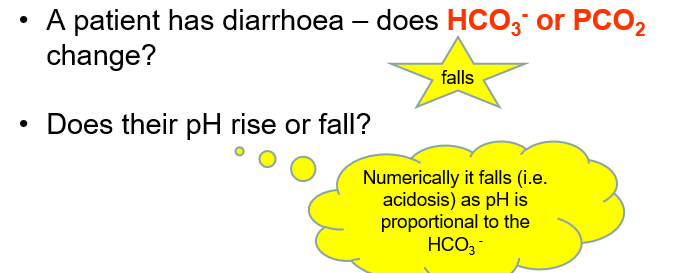
pH homeostasis can be disturbed if PaCO2 or the levels of HCO3- are not regulated in the body.
Respiratory and non-respiratory disturbances are pH imbalances that disrupt CO2 and HCO3- concentrations respectively.
Mixed conditions can co-exist too.
Respiratory and metabolic disturbances
Respiratory: Caused by CO2 being out of the normal range.
Metabolic: Those where electrolytes are out of normal range i.e HCO3- disturbances.
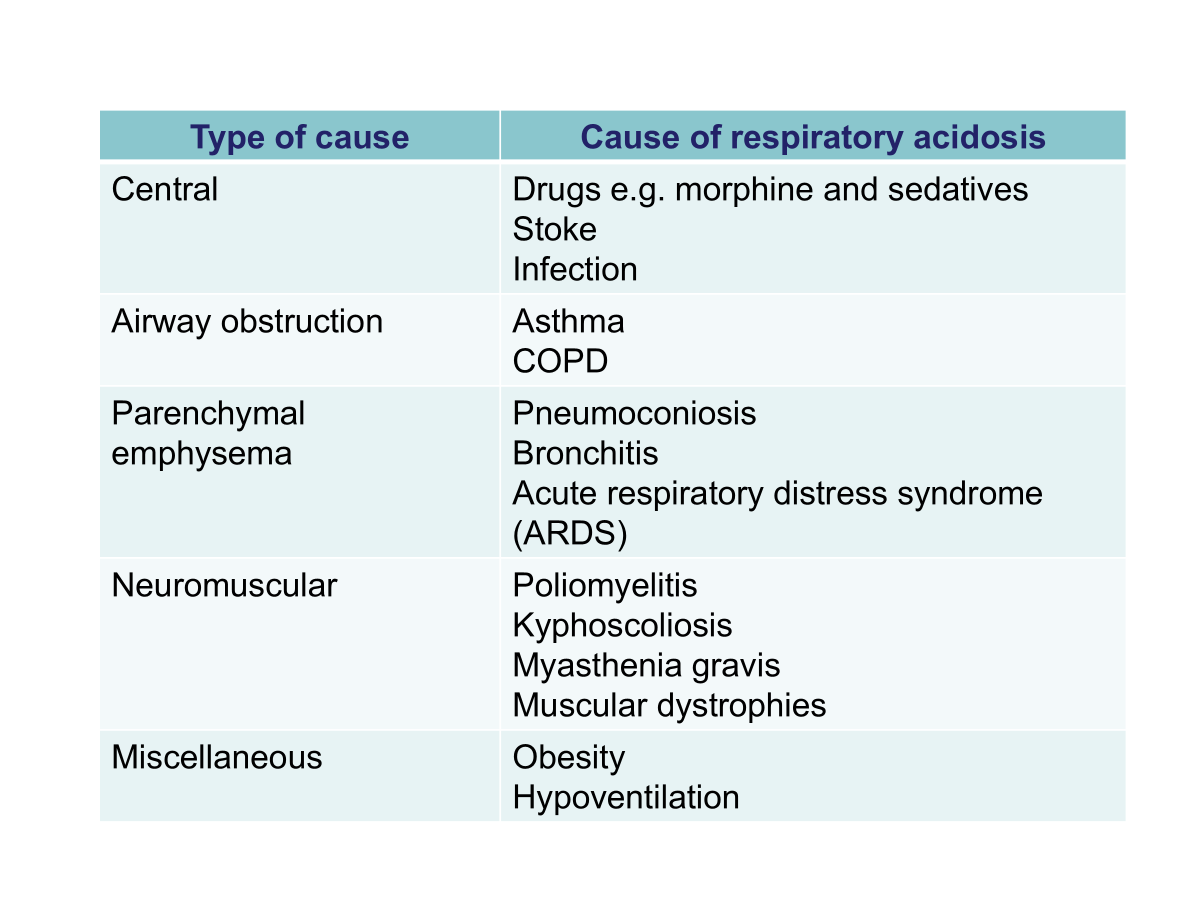
Respiratory Acidosis
High PCO2.
Due to decrease in gaseous exchange leading to CO2 retention.
Compensation by the kidneys results in increased H+ secretion over 3-5 days and an increased level of plasma bicarb ions.
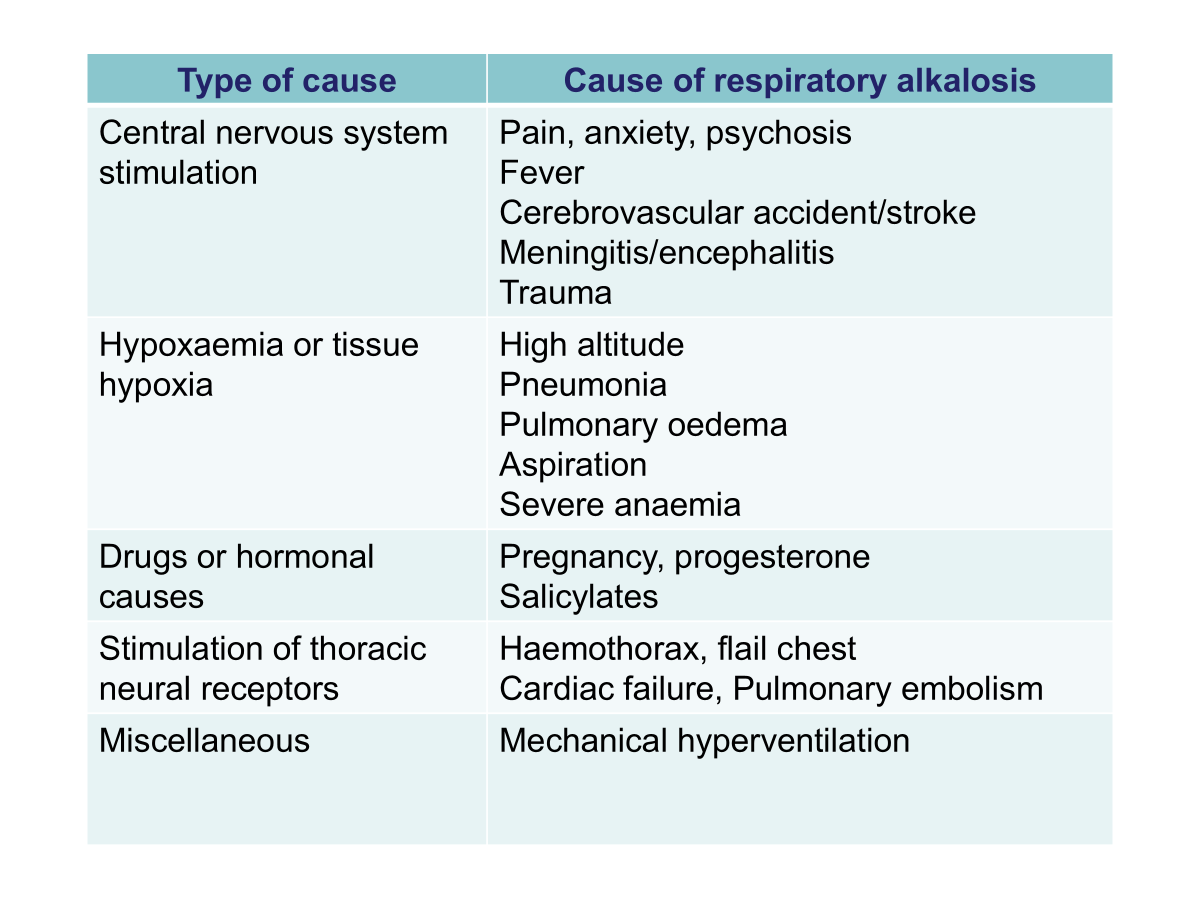
Respiratory Alkalosis
Low PCO2
Due to alveolar hyperventilation leading to excess removal of CO2.
Compensation by the kidneys results in a decrease in excretion of H+ and increased excretion of HCO3-.
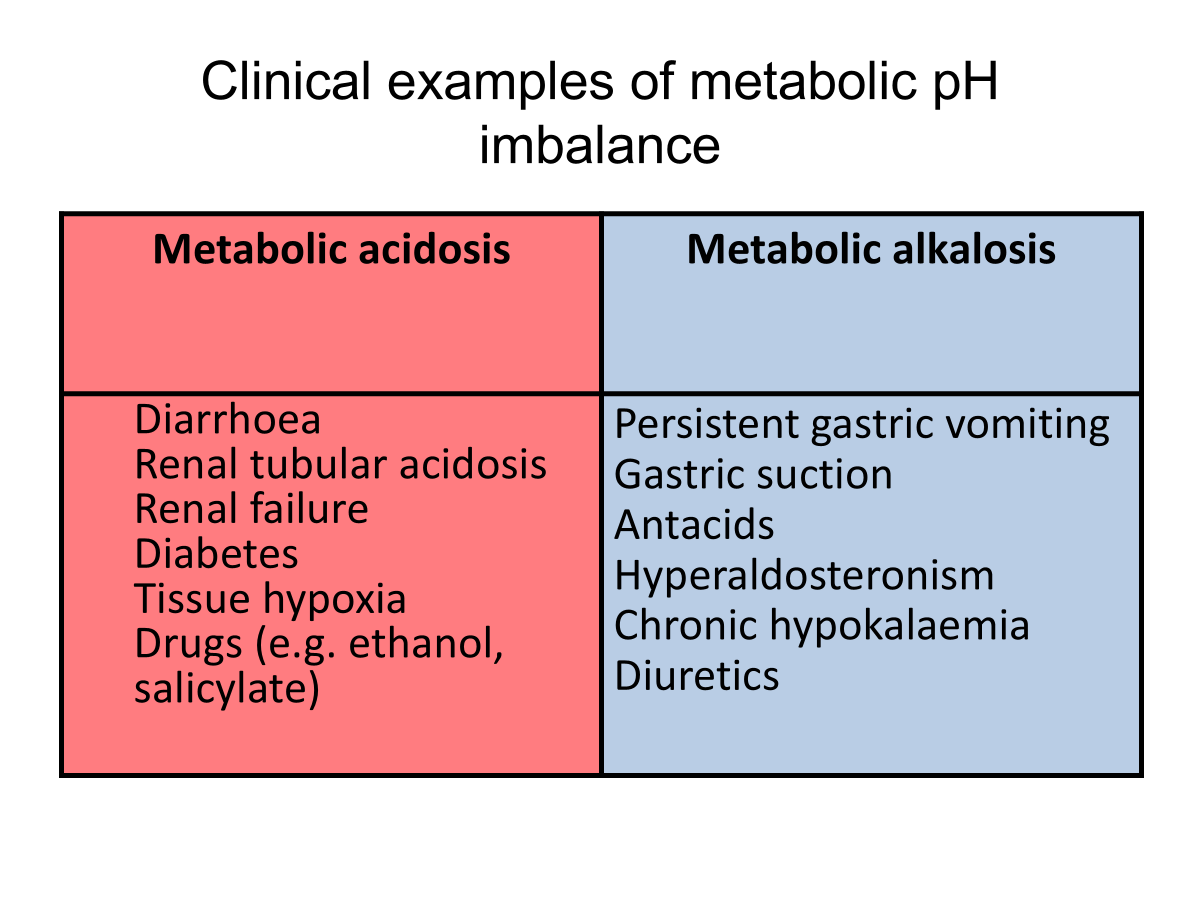
Clinical examples of metabolic pH imbalance
Arterial blood gas analysis
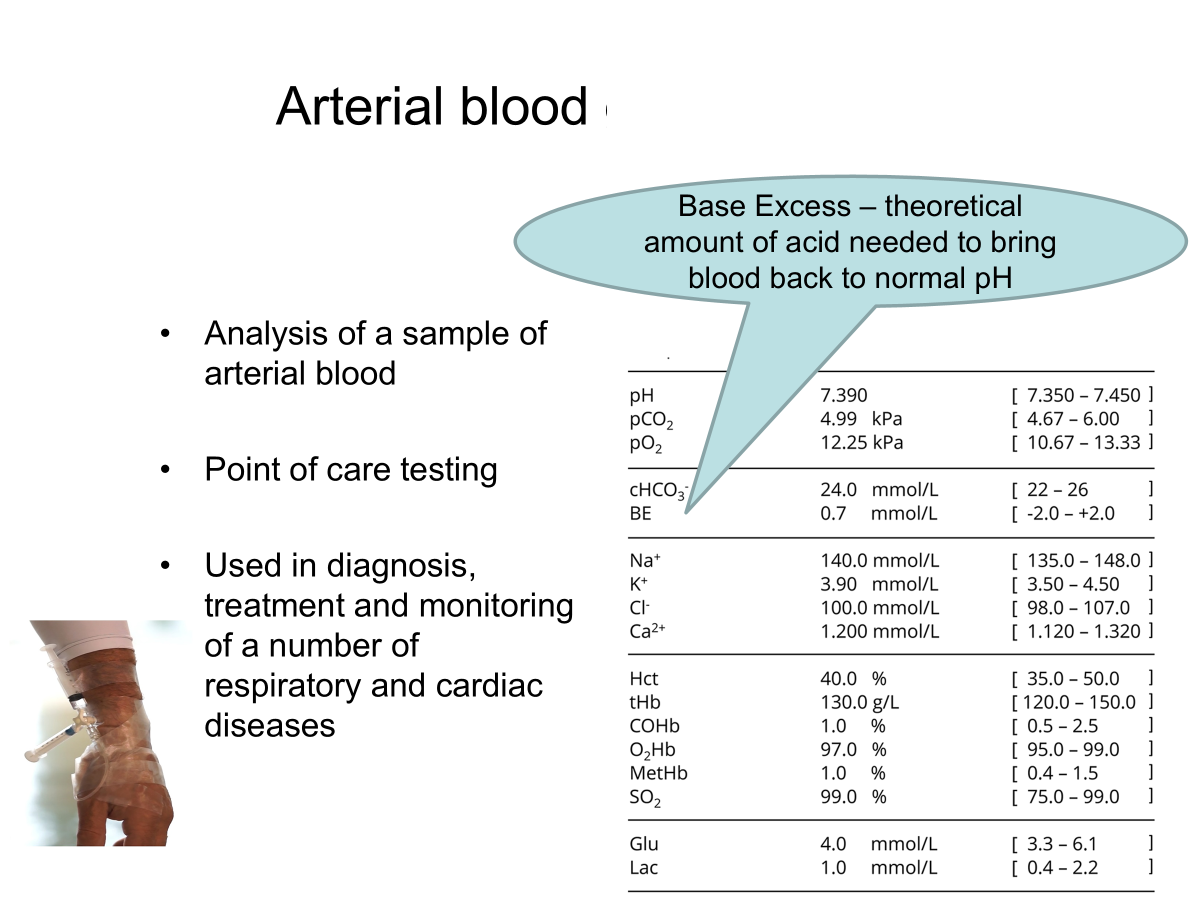
High BE means → metabolic alkalosis as there is a lot of acid needed to bring normality back.
Low BE means → metabolic acidosis as there is less acid needed to bring it back.
Respiratory cause: If CO2 is the main cause of the pH disturbance
Metabolic cause: If HCO3- is the main cause of the pH disturbance.
Compensation: physiological response to an acid-base imbalance
Correction: of the pH disturbance takes longer - kidneys.
Blood gas Analysis (BGA)
Is the pH normal? high or low?
Is PaCO2 or HCO3- out of their normal range.
Which of these abnormal results correspond with acid-base state?
e.g if pH is high this could be due to either a low PCO2 or high HCO3- , which one is seen in the data?
If the PaCO2 level corresponds with the pH, this is a respiratory cause. If the HCO3- is the culprit, it is a non-respiratory cause.
Look at the other if it is in the normal range, it is not yet compensation for the problem. If it is not in the normal range it is trying to correct the pH change.
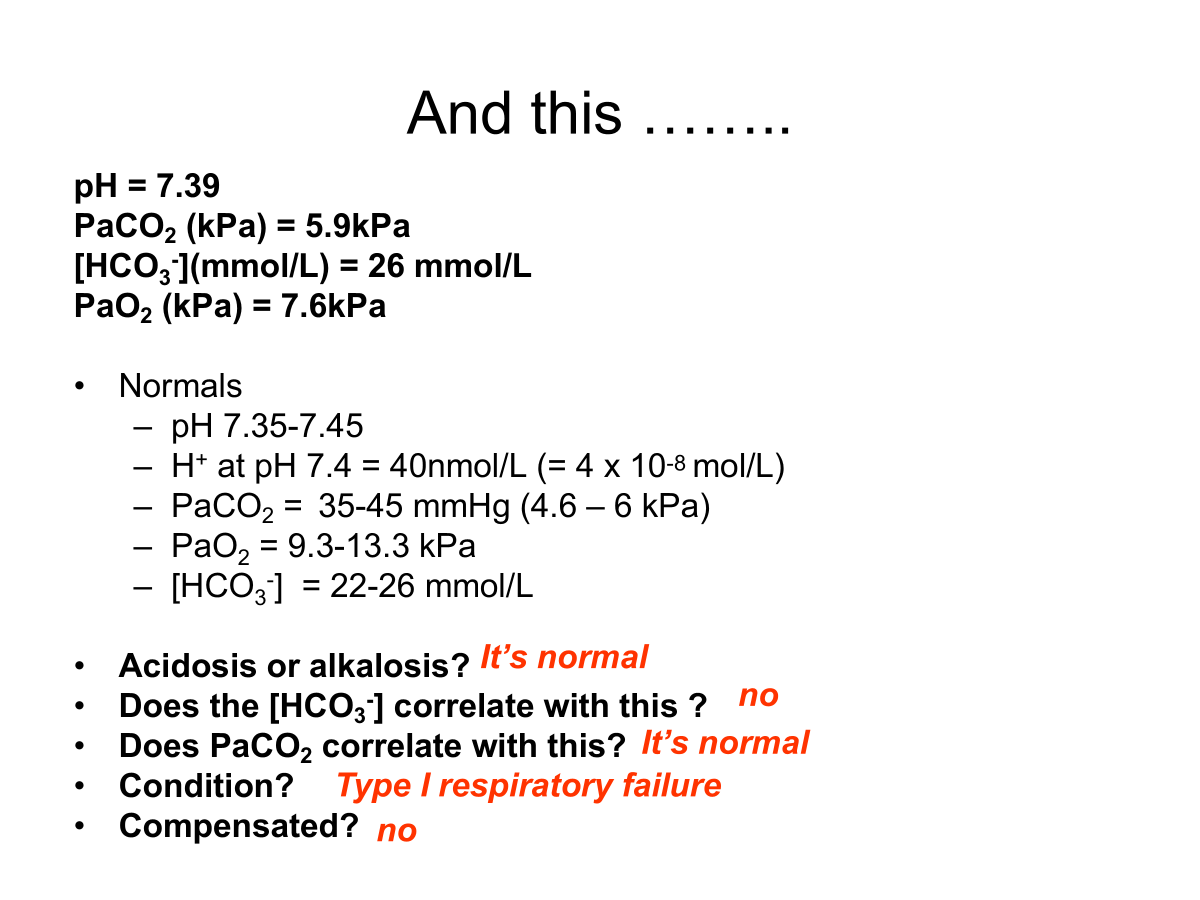
low PaCO2 → alkalosis
high HCO3- → alkalosis
= Respiratory alkalosis
Example Questions
Interpret the following ABG values:
pH = 7.31
PaCO₂ (kPa) = 7.2
[HCO₃⁻] (mmol/L) = 24 mmol/L
PaO₂ (kPa) = 10.1
Normals for reference:
pH = 7.35–7.45
PaCO₂ = 4.6–6.0 kPa
PaO₂ = 9.3–13.3 kPa
HCO₃⁻ = 22–26 mmol/L
Questions:
Acidosis or alkalosis? Acidosis
Does the HCO₃⁻ correlate with this? No → No metabolic compensation (not related to the acidosis)
Does the PaCO₂ correlate with this? Yes
Condition? Type 2 Respiratory Failure
Elevated PaCO2, acidosis and normal O2
Compensated? No
Interpret the following ABG values:
pH = 7.47
PaCO₂ (kPa) = 4.2
[HCO₃⁻] (mmol/L) = 20 mmol/L
PaO₂ (kPa) = 11.4
Normals for reference:
pH = 7.35–7.45
PaCO₂ = 4.6–6.0 kPa
PaO₂ = 9.3–13.3 kPa
HCO₃⁻ = 22–26 mmol/L
Questions:
Acidosis or alkalosis? Alkalosis
Does the HCO₃⁻ correlate with this? Yes
Does the PaCO₂ correlate with this? no
If the PaCO2 was low it should indicate acidosis as the PaCO2 would drop to compensate.
Condition? partially Compensated respiratory alkalosis
Compensated? Yes, partially
Interpret the following ABG values:
pH = 7.29
PaCO₂ (kPa) = 5.2
[HCO₃⁻] (mmol/L) = 18 mmol/L
PaO₂ (kPa) = 12.0
Normals for reference:
pH = 7.35–7.45
PaCO₂ = 4.6–6.0 kPa
PaO₂ = 9.3–13.3 kPa
HCO₃⁻ = 22–26 mmol/L
Questions:
Acidosis or alkalosis? Acidosis
Does the HCO₃⁻ correlate with this? Yes
Does the PaCO₂ correlate with this? No
Condition? Metabolic acidosis
Compensated? No
Low pH there is no evidence of PaCO2 dropping to compensate.
Interpret the following ABG values:
pH = 7.36
PaCO₂ (kPa) = 6.7
[HCO₃⁻] (mmol/L) = 30 mmol/L
PaO₂ (kPa) = 9.8
Normals for reference:
pH = 7.35–7.45
PaCO₂ = 4.6–6.0 kPa
PaO₂ = 9.3–13.3 kPa
HCO₃⁻ = 22–26 mmol/L
Questions:
Acidosis or alkalosis? Normal but leaning to acidosis
Does the HCO₃⁻ correlate with this? no
Does the PaCO₂ correlate with this? Yes
Condition? compensated respiratory acidosis
Compensated? Yes because the pH is still in normal range.
Interpret the following ABG values:
pH = 7.50
PaCO₂ (kPa) = 5.8
[HCO₃⁻] (mmol/L) = 32 mmol/L
PaO₂ (kPa) = 12.7
Normals for reference:
pH = 7.35–7.45
PaCO₂ = 4.6–6.0 kPa
PaO₂ = 9.3–13.3 kPa
HCO₃⁻ = 22–26 mmol/L
Questions:
Acidosis or alkalosis? Alkalosis
Does the HCO₃⁻ correlate with this? Yes
Does the PaCO₂ correlate with this? No but it is close to the high end
Condition? partially Compensated metabolic alkalosis
Compensated? Yes