Explaining Motion (OCR)
What is meant by matter?
Matter is anything that has mass and occupies space. It is composed of atoms and molecules, and it exists in various states such as solid, liquid, gas, and plasma.
Atomic Structure
Atoms:
The basic units of matter, consisting of a nucleus surrounded by electrons.
Nucleus:
Composed of protons (positively charged) and neutrons (neutral).
Electrons:
Negatively charged particles that orbit the nucleus in electron shells.
Atomic Number (Z):
The number of protons in the nucleus of an atom. This defines the element.
Mass Number (A):
The total number of protons and neutrons in the nucleus.
Isotopes:
Atoms of the same element with different numbers of neutrons.
States of Matter
Solids:
Particles are closely packed in a regular arrangement.
Strong intermolecular forces.
Fixed shape and volume.
Particles vibrate in fixed positions.
Liquids:
Particles are closely packed but can move past each other.
Weaker intermolecular forces than in solids.
Fixed volume but no fixed shape.
Particles can flow and take the shape of their container.
Gasses:
Particles are far apart and move freely.
Very weak intermolecular forces.
No fixed shape or volume.
Particles move randomly at high speeds.
Plasma:
High-energy state where electrons are stripped from atoms.
Consists of ions and free electrons.
Conducts electricity and is affected by magnetic fields.
Changes of State
Melting: Solid to liquid.
Freezing: Liquid to solid.
Vaporization: Liquid to gas (includes boiling and evaporation).
Condensation: Gas to liquid.
Sublimation: Solid to gas.
Deposition: Gas to solid
Properties of matter
Density (ρ)
Density is a measure of how much mass is contained in a given volume. It is a fundamental property that helps distinguish between different types of matter.
Formula: p = m / v
Buoyancy: Objects with lower density than the fluid they are in will float.
Pressure (P)
Pressure is the force exerted per unit area on the surface of an object. It plays a crucial role in understanding how gasses and liquids behave under different conditions.
Formula: P = F/A
Temperature (T)
Temperature is a measure of the average kinetic energy of the particles in a substance. It is a fundamental property that affects the state and behavior of matter.
Scales: Celsius (°C), Fahrenheit (°F), and Kelvin (K).
Relationship with Kinetic Energy: Higher temperature means higher average kinetic energy of particles.
Volume (V)
Volume is the amount of space that a substance or object occupies.
Units: Cubic meters (m³), liters (L), milliliters (mL), and cubic centimeters (cm³).
Elasticity and Plasticity
Elasticity: The ability of a material to return to its original shape after being deformed.
Young’s Modulus (E): Measures the stiffness of a material.
Plasticity: The ability of a material to undergo permanent deformation.
Atomic Models and Theories
1.Dalton’s Atomic Theory (Early 19th Century)
John Dalton proposed the first modern atomic theory based on experimental evidence. His theory laid the foundation for our understanding of matter.
Key Features:
Matter is composed of indivisible atoms.
All atoms of a given element are identical in mass and properties.
Compounds are formed by the combination of atoms of different elements in fixed ratios.
Chemical reactions involve the rearrangement of atoms; atoms are neither created nor destroyed in these reactions.
Limitations:
Did not explain the internal structure of the atom.
Did not account for isotopes (atoms of the same element with different masses).
2. Thomson’s Model (1897)
J.J. Thomson discovered the electron through his experiments with cathode rays, leading to the "plum pudding" model of the atom.
Key Features:
Atoms are composed of a positively charged "pudding" with negatively charged electrons embedded within it, like plums in a pudding.
The overall charge of the atom is neutral.
Limitations:
Did not explain the arrangement of electrons.
Failed to account for the nucleus of the atom.
3. Rutherford’s Model (1911)
Ernest Rutherford’s gold foil experiment led to the discovery of the atomic nucleus.
Key Experiment:
Alpha particles were directed at a thin gold foil. Most particles passed through, but some were deflected at large angles.
Key Features:
Atoms consist of a small, dense, positively charged nucleus at the center.
Electrons orbit the nucleus, similar to planets orbiting the sun.
Most of the atom’s volume is empty space.
Limitations:
Could not explain the stability of the electron orbits.
Did not explain atomic spectra.
4. Bohr’s Model (1913)
Niels Bohr improved upon Rutherford’s model by introducing quantized electron orbits.
Key Points:
Quantized Orbits: Electrons orbit the nucleus in specific, quantized energy levels.
Energy Absorption/Emission: Electrons can move between energy levels by absorbing or emitting photons of specific energies.
Stability: Electrons in quantized orbits do not radiate energy and thus are stable.
Limitations: It could not accurately predict spectra for atoms with more than one electron.
5. Quantum Mechanical Model (1920s - Present)
The development of quantum mechanics led to a more accurate and sophisticated model of the atom.
Key Contributors: Erwin Schrödinger, Werner Heisenberg, Paul Dirac, and others.
Key Points:
Wave-Particle Duality: Electrons exhibit both wave-like and particle-like properties.
Orbitals: Electrons occupy probabilistic regions of space called orbitals, rather than fixed paths.
Uncertainty Principle: Heisenberg’s Uncertainty Principle states that it is impossible to simultaneously know both the exact position and exact momentum of an electron.
Schrödinger’s Equation: Describes how the quantum state of a physical system changes over time.
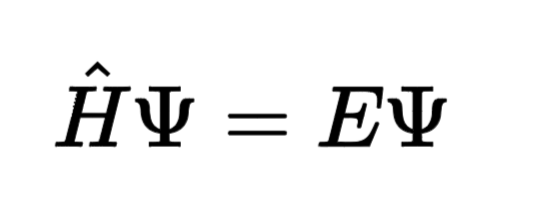
H^\hat{H}H^ is the Hamiltonian operator (total energy operator).
ψ\psiψ is the wavefunction of the system.
EEE is the energy eigenvalue.
Electron Configurations: Describes the arrangement of electrons in an atom’s orbitals.
The Periodic Table
Groups:
Vertical columns in the periodic table, indicating elements with similar properties and the same number of valence electrons.
Periods:
Horizontal rows, indicating the number of electron shells.
Bonding and Interactions
Ionic Bonding:
Transfer of electrons from one atom to another, resulting in the formation of ions.
Covalent Bonding:
Sharing of electrons between atoms.
Metallic Bonding:
Electrons are shared in a "sea" of electrons, allowing metals to conduct electricity and heat.
Intermolecular Forces:
Forces between molecules, including Van der Waals forces, dipole-dipole interactions, and hydrogen bonding.