Measurement and Data Processing (IB)
11.1 Uncertainties and Errors in Measurements and Results
Qualitative & Quantitative Analysis
Analysis of data can be classified into two types:
Qualitative Analysis: Comes from observations and non-numerical methods
Quantitative Analysis: Comes from measurements and is always associated with uncertainties determined by either the apparatus or human limitations such as reaction times and sight.
Uncertainties
In science, numerical data is divided into two types:
1. Data with exact numbers (no uncertainty)
2. Data with inexact numbers (Degree of uncertainty involved)
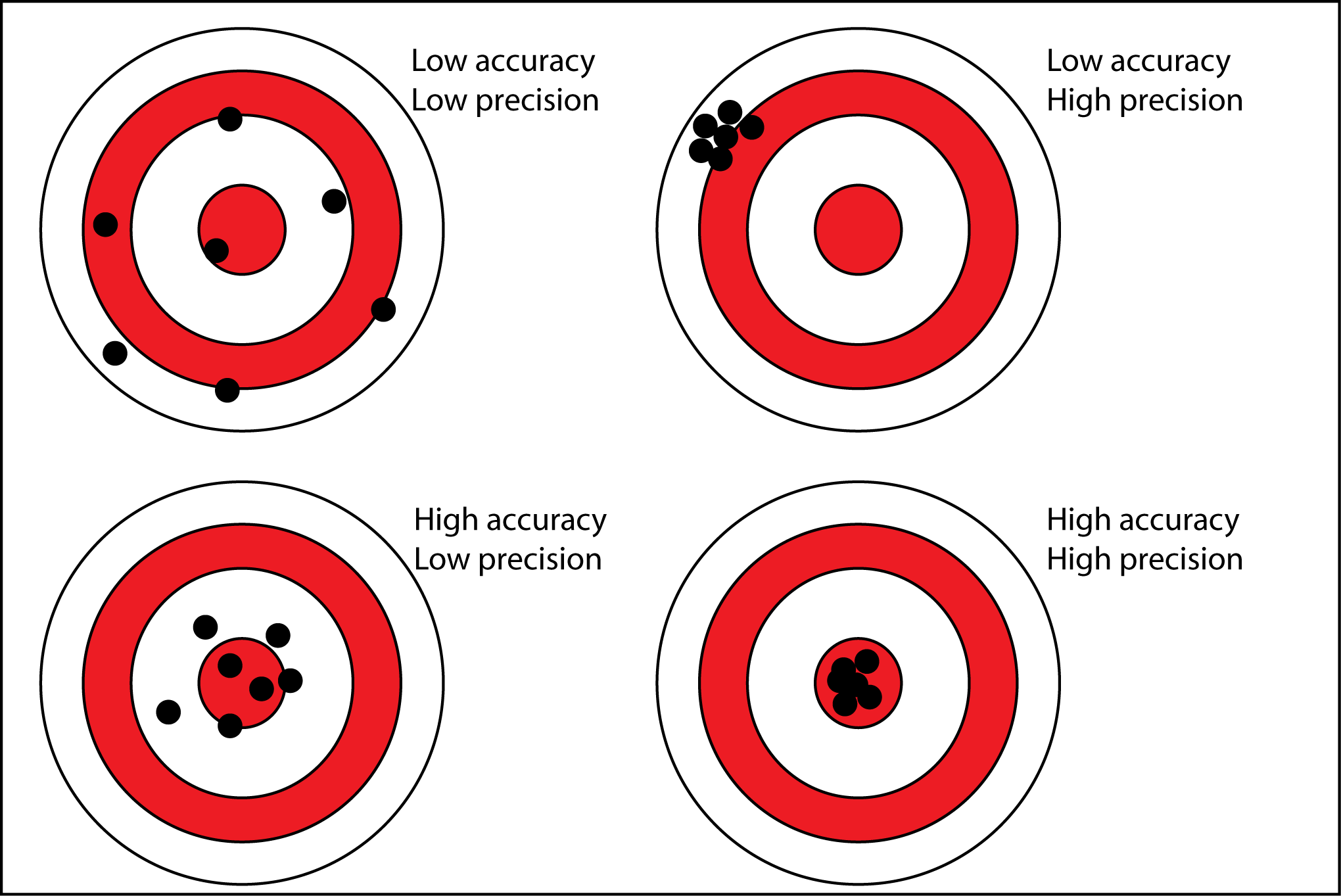
Precision vs Accuracy
Precision: Closeness of agreement between interdependent test results
Accuracy: Closeness of agreement between the result of measurement and the true value
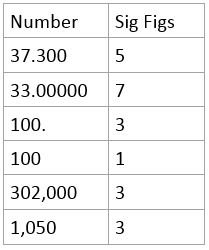
Significant Figures
Number of digits reflecting the precision of a given measurement
Sometimes it is useful to write in Scientific Notation
Here are some examples:
Some rules for significant figures in calculation
For multiplication or division: The result should be expressed based on measurement with the smallest number of significant figures.
For addition or subtraction: The result should be expressed based on measurement with the smallest number of decimal places
(A simple rule on the side is that your final answer should be expressed in the number of significant figures of the measurement with the lowest number of significant figures)
Experimental Error
Every measurement has a degree of uncertainty called experimental error.
There are two types of experimental error
Systematic error: A flaw in experimental design or methodology. This affects the accuracy of the results.
Random error:
-
Absolute and Relative Uncertainty
Absolute Uncertainty is the margin of uncertainty associated with the result of a measurement.
Its symbol is given by ΔA
Relative Uncertainty is the ratio comparing the size of the absolute uncertainty.
Relative uncertainty = ΔA / A
All experimental results should be reported in the form:
Experimental result = (A ± ΔA) units
Percentage Error
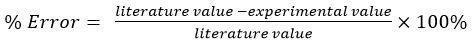
11.2 Graphical Techniques
Graphical techniques: an effective means of communicating the effect of an independent variable on a dependent variable, and can lead to the determination of physical quantities.
Sketched graphs have labeled but unscaled axes.
They are used to show qualitative trends, such as variables that are proportional or inversely proportional.
Units generally would not need to be shown on a sketch, only the variables.
Drawn graphs have labeled and scaled axes.
They are based on quantitative measurements.
Drawn graphs always display the appropriate units for variables.
Dependence is considered any statistical relationship between two sets of data or between two random variables.
In a graph of Y versus X, the independent variable (that is, the cause) is plotted on the x-axis, and the dependent variable (the effect) is plotted on the y-axis.
Correlation can be described as a statistical measure and technique that indicates the degree and direction of the relationship between two sets of variables.
A positive correlation is where the two variables increase or decrease in parallel to one another.
A negative correlation is one in which one variable increases while the second variable decreases or vice versa.
Correlations can be deduced from the correlation coefficient, represented by the symbol, r.
This coefficient is a measure of the strength of the relationship between two variables. Data are often represented by scatter plots that show the scatter of various points on a graph.
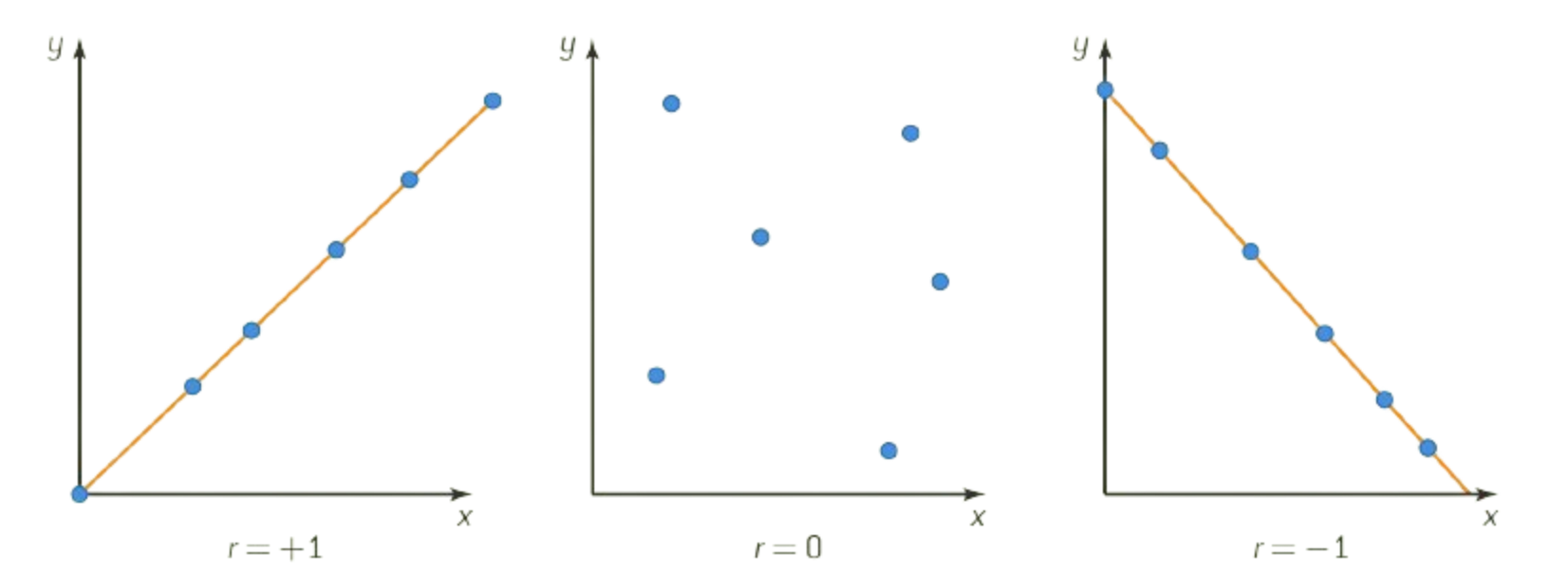
r = +1, is indicative of a perfect positive linear relationship (all points lie on a straight line)
r = 0, no linear relationship exists (there is a complete scatter of points)
r = -1, is indicative of a perfect negative linear relationship
Slope (m): the tangent of the angle θ, that the line makes with the positive direction of the x-axis.
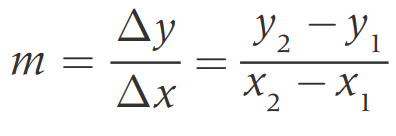
Intercept (c): points where the line cuts the y-axis at x=0
Can be found by→
Extrapolation
Equation of a line [y = mx + b]
Best Fit Line: a straight line that minimizes the distance between it and some data.
11.3 Spectroscopic Identification of Organic Compounds
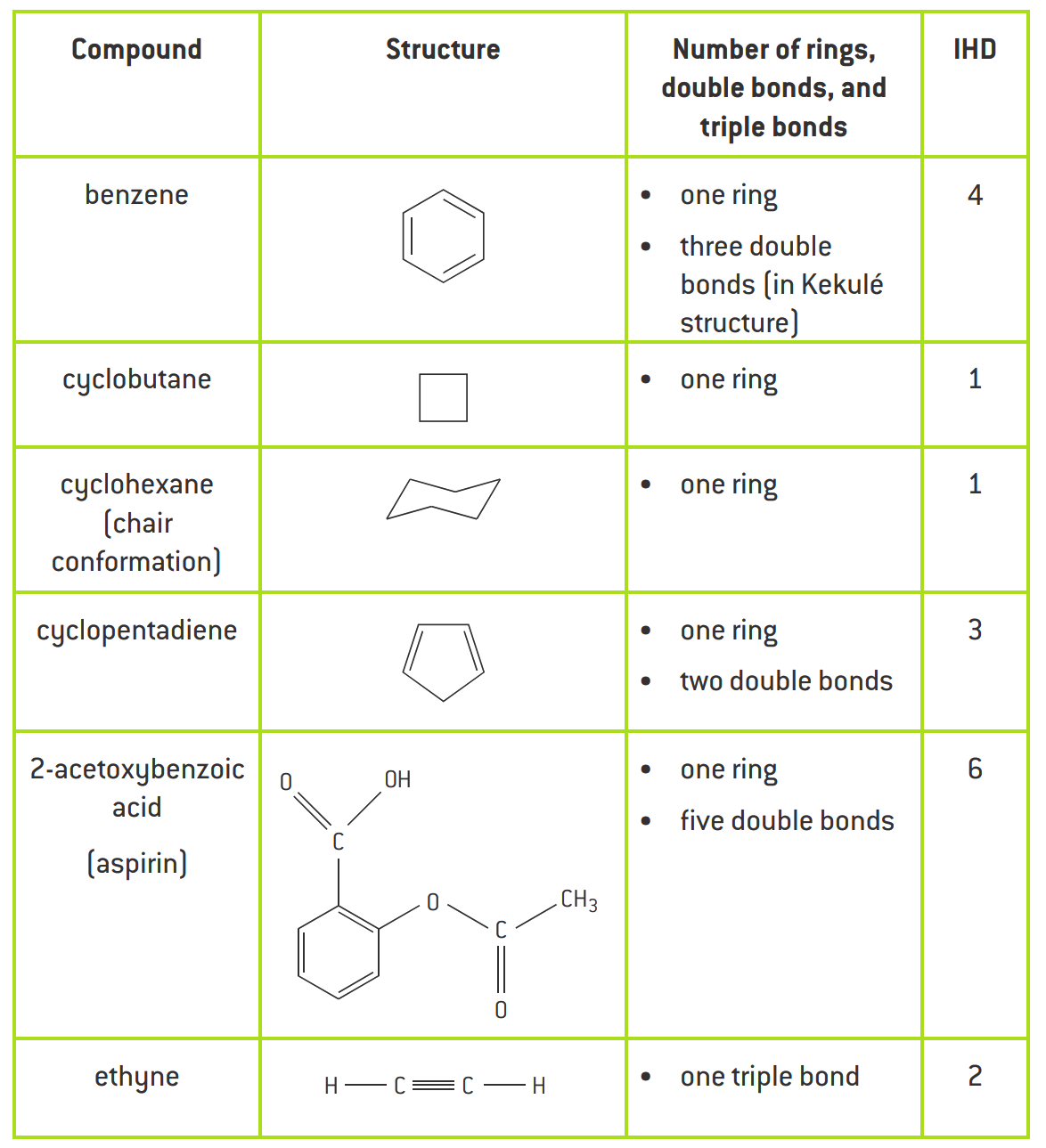
Degree of unsaturation or index of hydrogen deficiency (IHD)
Used to determine the number of rings or double bonds from a molecular formula
Double bond = 1 IHD
Triple bond = 2 IHD
Ring = 1 IHD
Aromatic = 4 IHD (3 double bond + 1 ring)
IHD from Structure
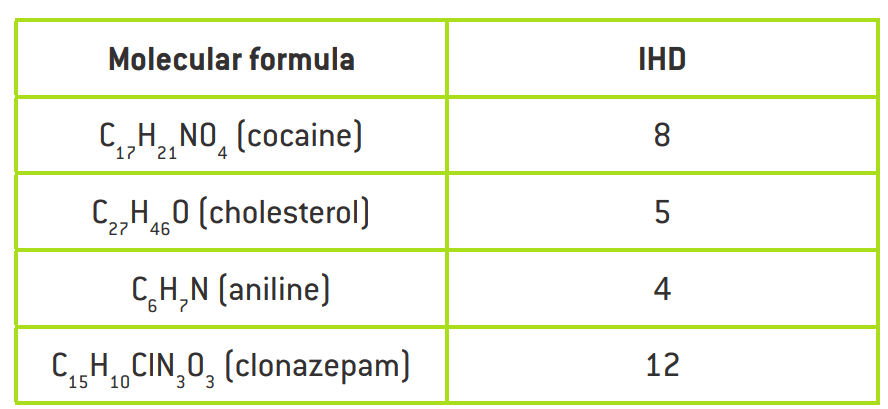
IHD from Molecular formula
For the generic formula CcHhNnOoXx:
IHD = (0.5)(2c + 2 – h – x + n)
Ex:
Electromagnetic Spectrum
Various regions of EMS are the basis of different types of spectroscopy
The energy of electromagnetic radiation, E, is related to the frequency v of the radiation by Planck equation:
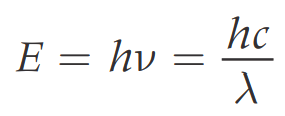
h = Plancks constant = 6.63 x 10 -34 J s
E = energy of radiation (measured in J)
v = frequency of radiation (measured in Hz)
c = speed o light = 3.00 x 108 m s-1
λ = wavelength (measured in m)
Techniques used to identify structures of substances
X-rays – as their energy is high, these cause electrons to be removed from the inner energy levels of atoms. Direction patterns can lead to information such as the bond distances and bond angles in a structure and form the basis of X-ray crystallography.
Visible and UV light–electronic transitions and hence this type of spectroscopy gives information about the electronic energy levels in an atom or molecule. This is the basis of UV-vis spectroscopy.
Infrared Radiation – causes certain bonds in a molecule to vibrate (for example, stretch and bend) and as such provides information on the functional groups present. This is the basis of IR spectroscopy.
Microwaves – cause molecular rotations and can give information on bond lengths.
Radiowaves – can cause nuclear transitions in a strong magnetic field because radiowaves can be absorbed by certain nuclei, which causes their spin states to change. Nuclear magnetic resonance (NMR) spectroscopy is based on this and information on different chemical environments of atoms can be deduced, which leads to information on the connectivity of the atoms present in a molecule.
Spectroscopy
Infrared Spectroscopy
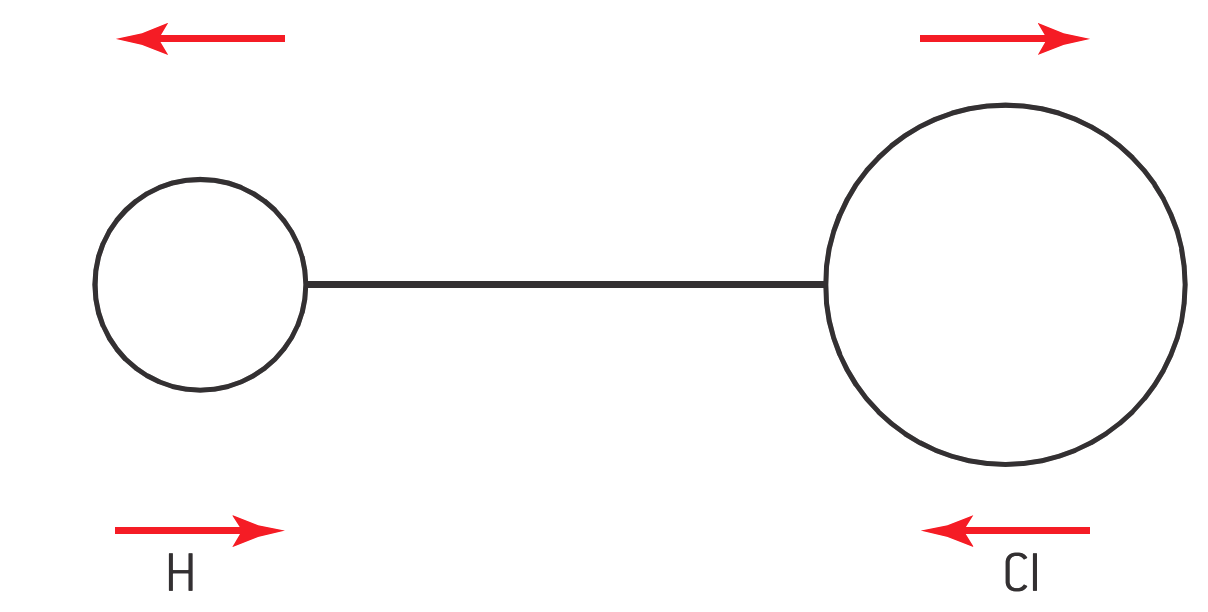
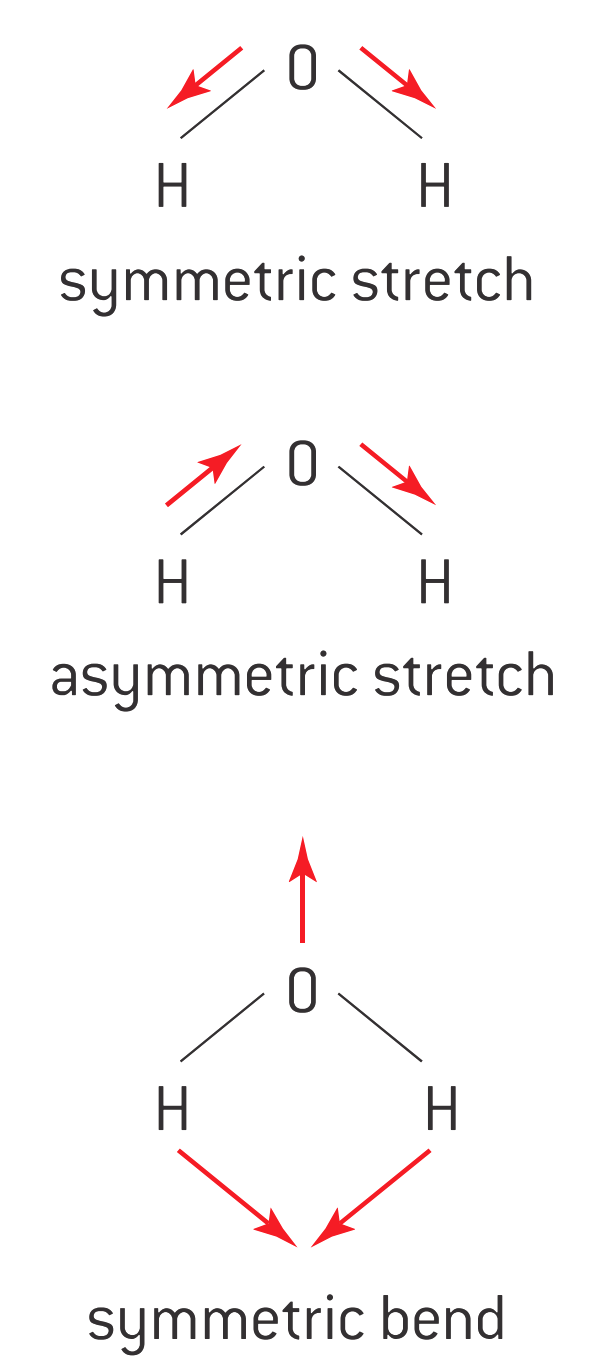
IR radiation does not have sufficient energy to result in electronic transitions but can cause molecular vibrations, which result in the vibration of certain groups of molecules about their bonds. The basis of IR spectroscopy is the spring model.
In the spring model, every covalent bond is considered as a spring. Such a spring can be stretched (both symmetrically and asymmetrically), bent, or twisted, giving rise to a distortion.
Hookes law: The force required to cause the vibration.
k = spring constant
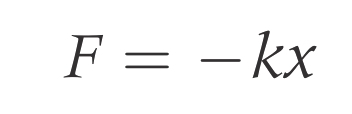
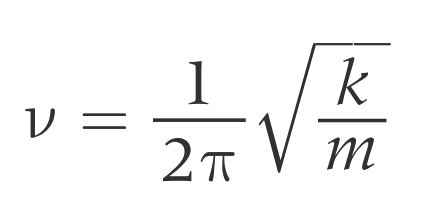
Atom’s Vibration
Lighter atoms vibrate at higher frequencies (v).
Heavier atoms vibrate at lower frequencies (v).
Multiple bonds (e.g., double and triple bonds) follow the same trend as lighter atoms.
Different molecules absorb at different frequencies due to variations in bond enthalpy.
IR absorptions are typically reported in wavenumber (1/λ), measured in cm-1.
Proton Nuclear Magnetic Resonance (1H NMR) Spectroscopy
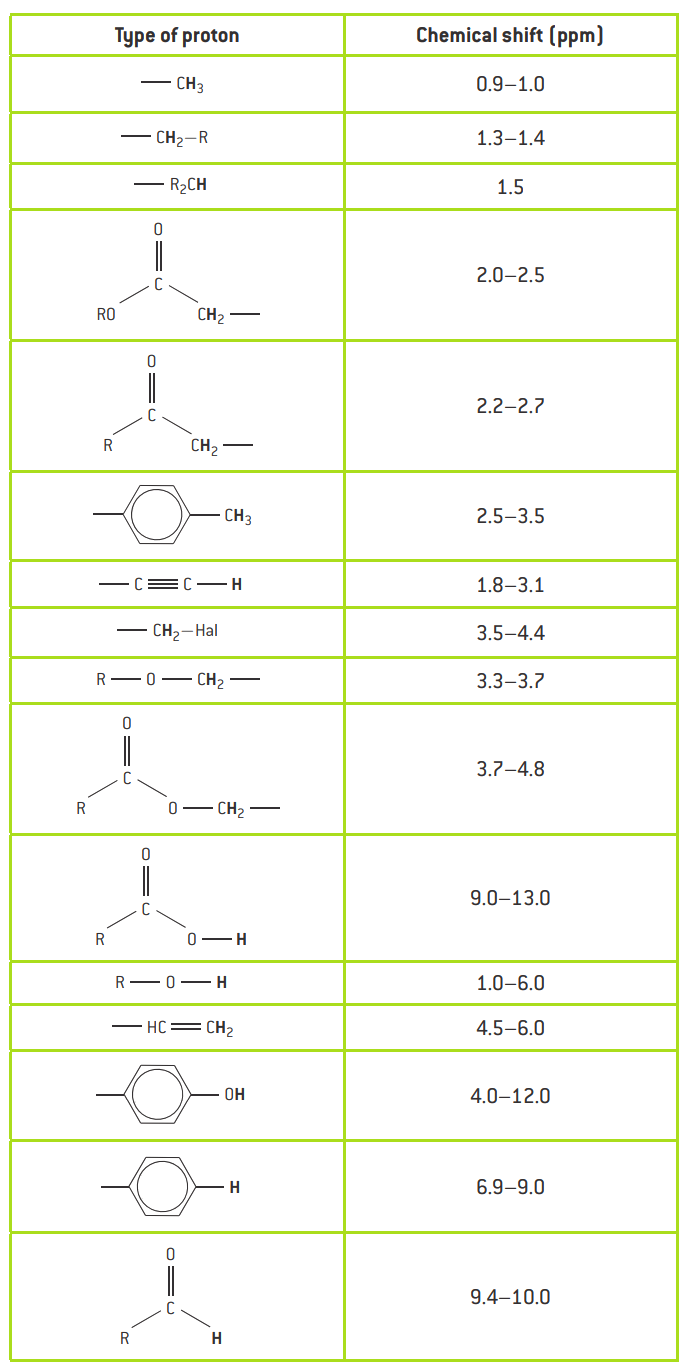
Provides information about the different chemical environments of hydrogen atoms in a molecule.
Based on the principle that the nuclei of hydrogen atoms have two possible spin states and act like tiny magnets.
The position of the spin relative to the standard spin (tetramethylsilane, TMS) is called the chemical shift.
Gives information about the relative number of hydrogens in each environment, presented as a ratio.
The area under the curve in a spectrum corresponds to the number of hydrogens in that environment.
Mass Spectroscopy
Provides additional information about functional groups in a molecule.
When a gaseous molecule is ionized, a molecular ion (M⁺) is formed.
The fragmentation pattern in a mass spectrum shows the masses of fragments after functional groups are removed.
The molecular ion peak in a mass spectrum represents the molecular mass of the compound.