19 Polymers
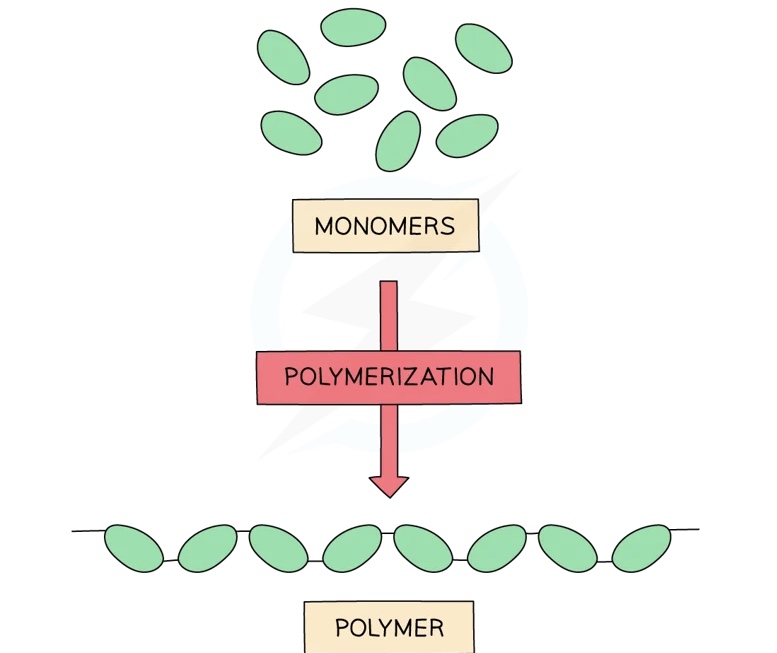
Polymers are large molecules built by linking 50 or more smaller molecules called monomers
Each repeat unit is connected to the adjacent units via covalent bonds
Some polymers contain just one type of unit
- Examples include poly(ethene) and poly(chloroethene), commonly known as PVC
Others contain two or more different types of monomer units and which are called copolymers
- Examples include nylon and biological proteins
Different linkages also exist, depending on the monomers and the type of polymerisation
- Examples of linkages are covalent bonds, amide links and ester links

Plastic and man made fibers
Synthetic polymers are ones made in a factory, for example nylon, terylene and lycra
Nylon is a polyamide used to produce clothing, fabrics, nets and ropes
Terylene is a polyester made from monomers which are joined together by ester links
Terylene is used extensively in the textile industry and is often mixed with cotton to produce clothing
Non Biodegradable Plastics
- These are plastics which do not degrade over time or take a very long time to degrade, and cause significant pollution problems
- In particular plastic waste has been spilling over into the seas and oceans and is causing huge disruptions to marine life
- In landfills waste polymers take up valuable space as they are non-biodegradable so microorganisms cannot break them down. This causes the landfill sites to quickly fill up
- Polymers release a lot of heat energy when incineratedand produce carbon dioxide which is a greenhouse gas that contributes to climate change
- If incinerated by incomplete combustion, carbon monoxidewill be produced which is a toxic gas that reduces the capacity of the blood to carry oxygen
- Polymers can be recycled but different polymers must be separated from each other which is a difficult and expensive process
Addition & Condensation Polymers & Deducing Structures

Addition Polymerization
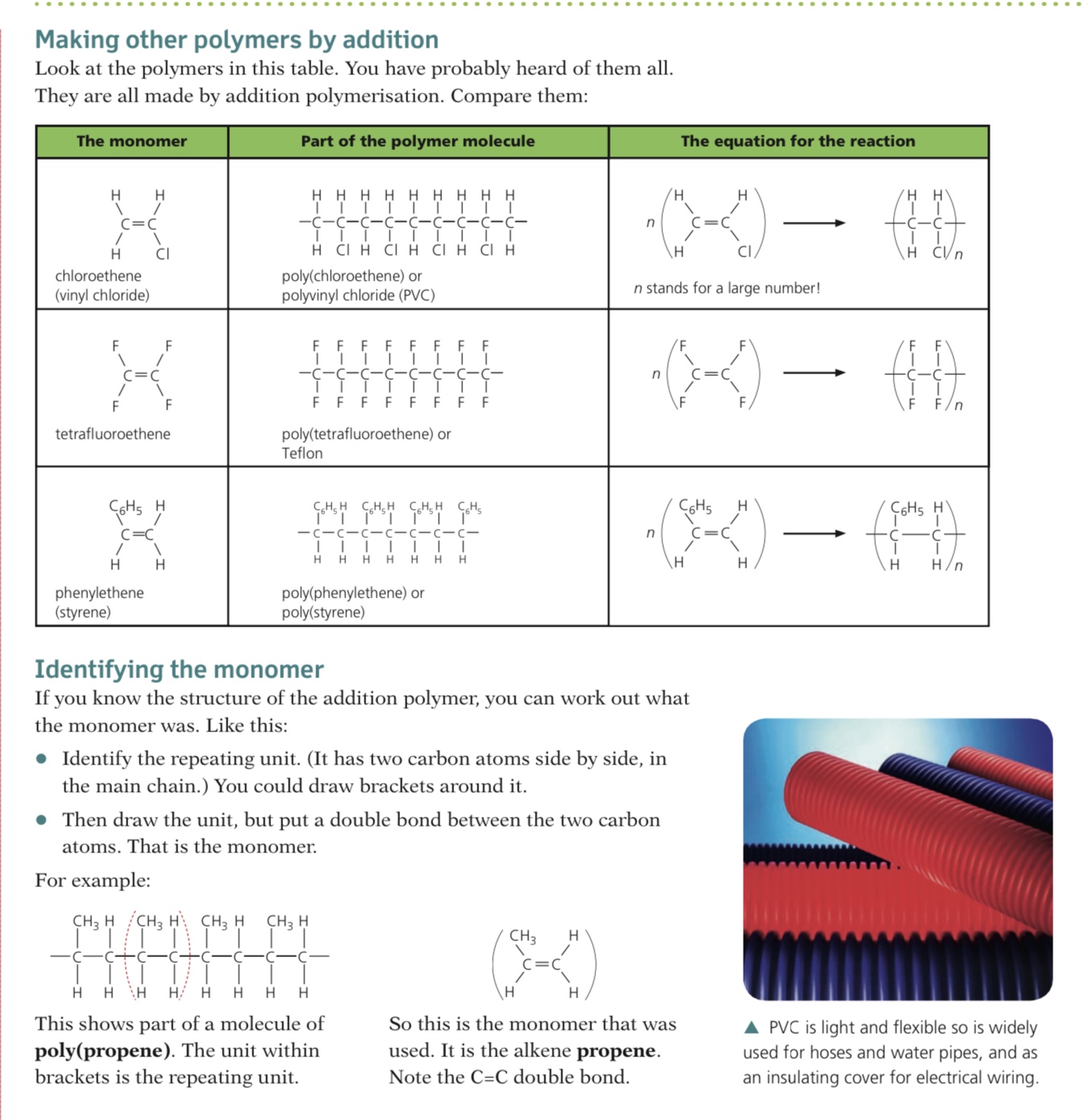
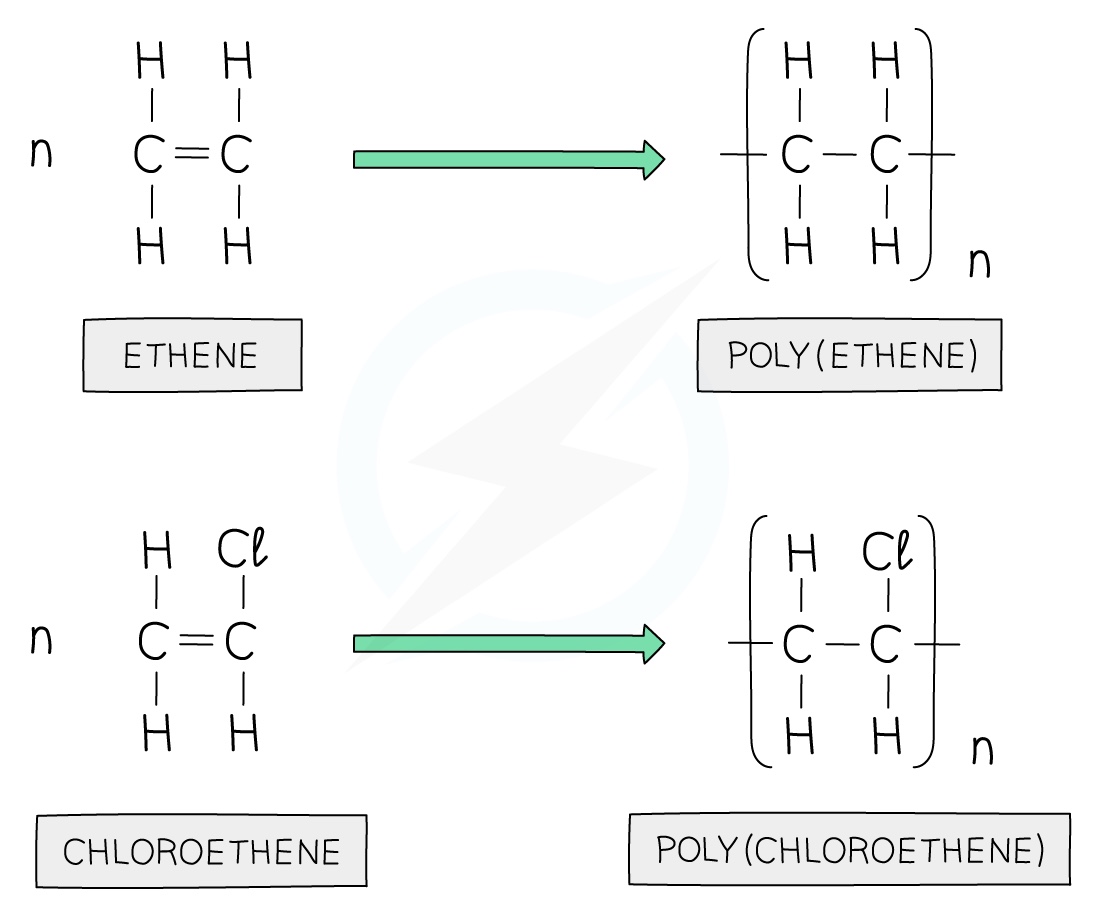
Addition polymers are formed by the joining up of many monomers and only occurs in monomers that contain C=C bonds
One of the bonds in each C=C bond breaks and forms a bond with the adjacent monomer with the polymer being formed containing single bonds only
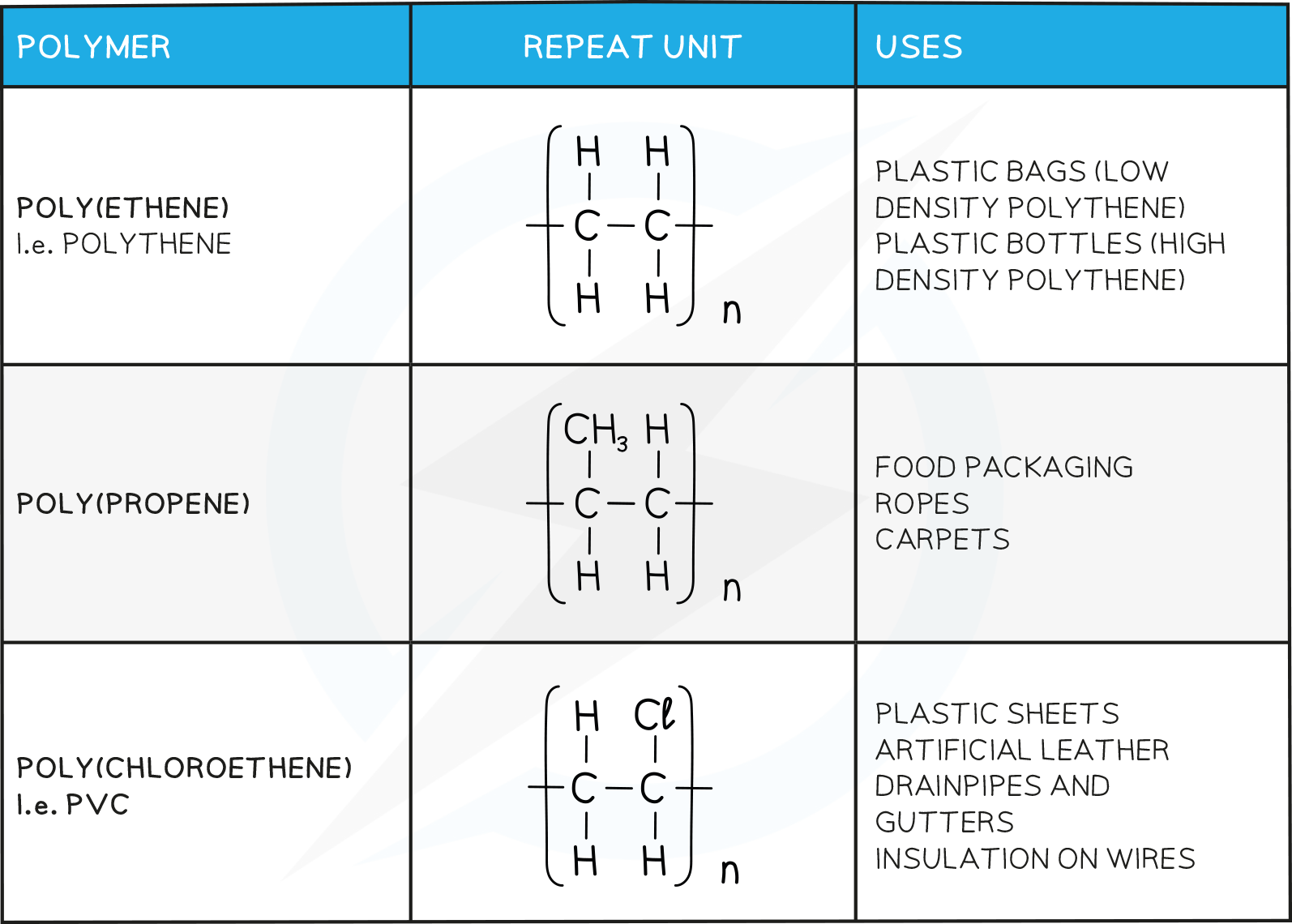
Many polymers can be made by the addition of alkene monomers
Others are made from alkene monomers with different atoms attached to the monomer such as chlorine or a hydroxyl group
The name of the polymer is deduced by putting the name of the monomer in brackets and adding poly- as the prefix
For example if propene is the alkene monomer used, then the name is polypropene
Polyethene is formed by the addition polymerisation of ethene monomers
Deducing the polymer from the monomer
- Polymer molecules are very large compared with most other molecule
- Repeat units are used when displaying the formula
- To draw a repeat unit, change the double bond in the monomer to a single bond in the repeat unit
- Add a bond to each end of the repeat unit
- The bonds on either side of the polymer must extend outside the brackets (these are called extension or continuation bonds)
- A small subscript n is written on the bottom right hand side to indicate a large number of repeat units
- Add on the rest of the groups in the same order that they surrounded the double bond in the monomer
Deducing the monomer from the polymer
- Identify the repeating unit in the polymer
- Change the single bond in the repeat unit to a double bond in the monomer
- Remove the bond from each end of the repeat unit

Condensation Polymerization
Condensation polymers are formed when two different monomers are linked together with the removal of a small molecule, usually water
This is a key difference between condensation polymers and addition polymers:
- Addition polymerisation forms the polymer molecule only
- Condensation polymerisation forms the polymer molecule and one water molecule per linkage
The monomers have two functional groups present, one on each end
The functional groups at the ends of one monomer react with the functional group on the end of the other monomer, in so doing creating long chains of alternating monomers, forming the polymer
Hydrolysing (adding water) to the compound in acidic conditions usually reverses the reaction and produces the monomers by rupturing the peptide link

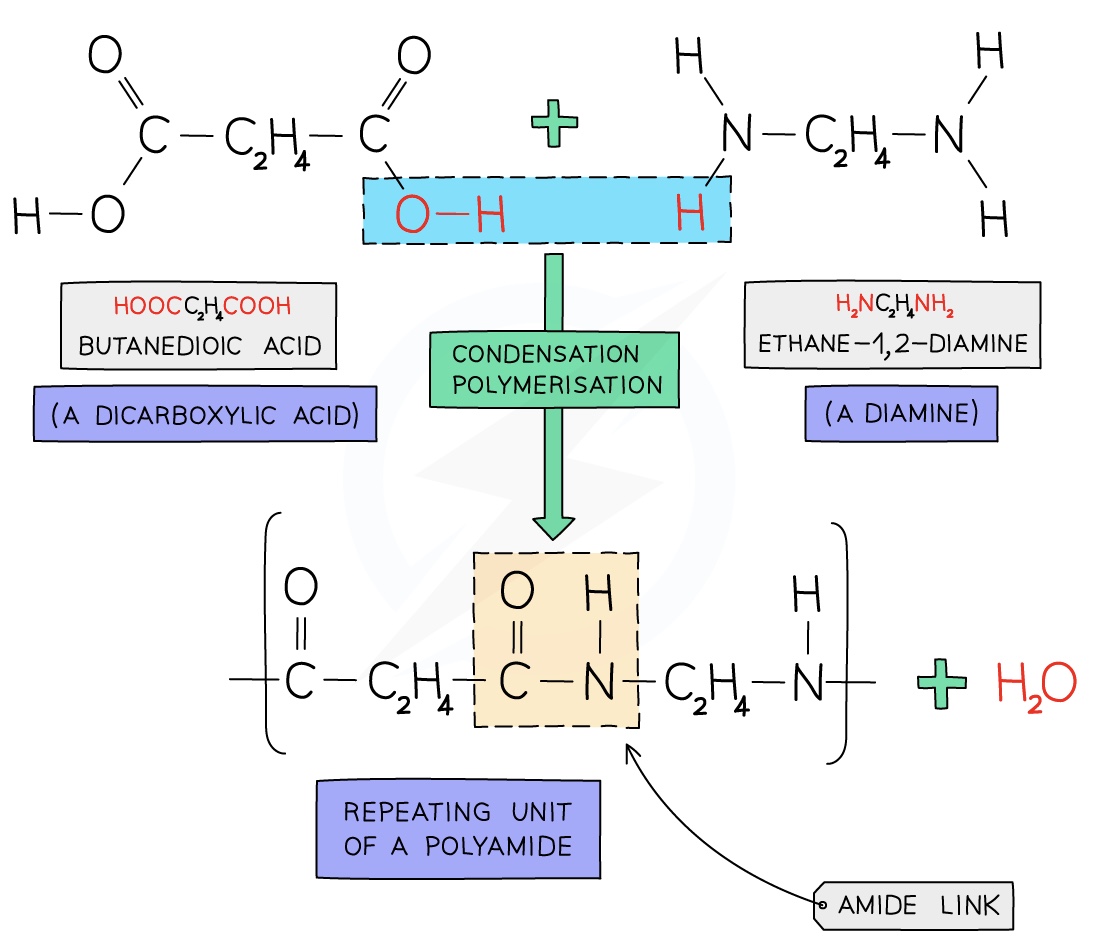
Forming Nylon
Nylon is a polyamide made from dicarboxylic acid monomers (a carboxylic with a -COOH group at either end) and diamines (an amine with an -NH2 group at either end)
Each -COOH group reacts with another -NH2 group on another monomer
An amide linkage is formed with the subsequent loss of one water molecule per link
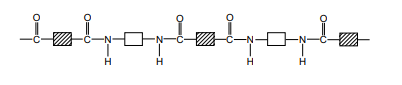
The structure of nylon can be represented by drawing out the polymer using boxes to represent the carbon chains
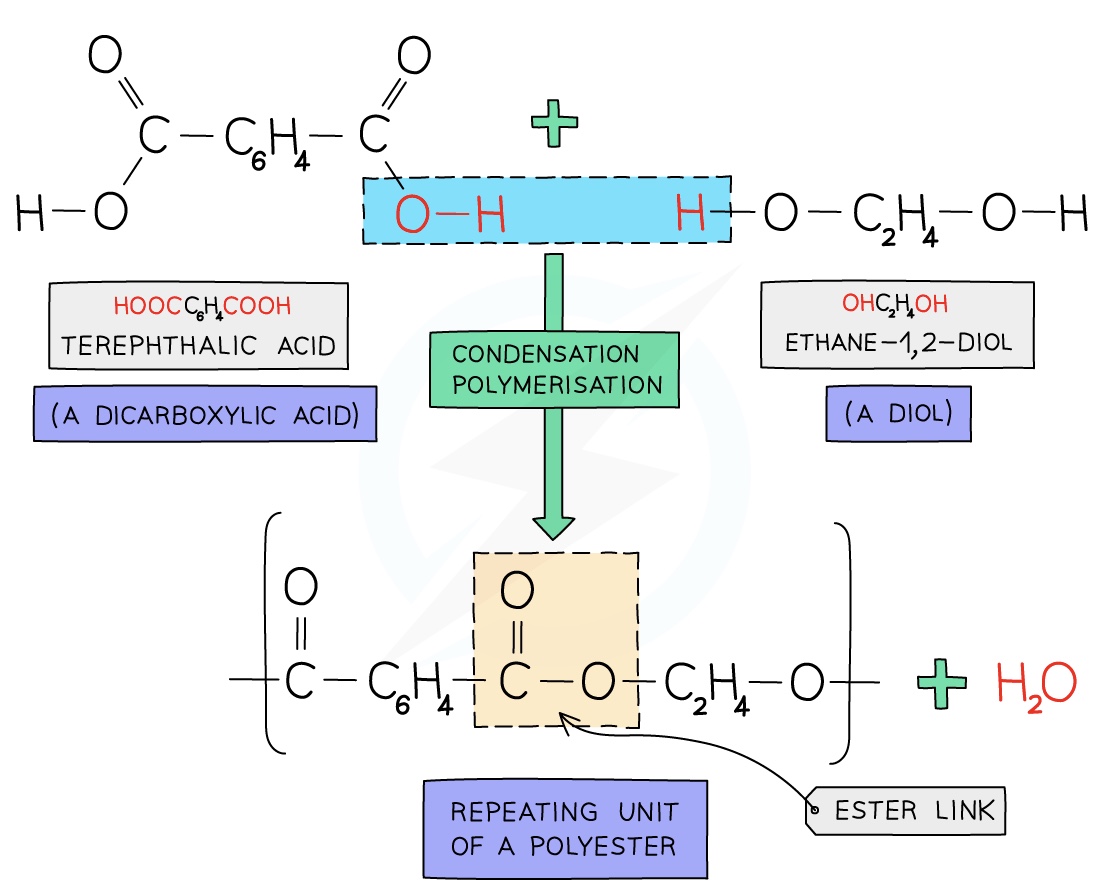
Formation of Terylene
Terylene is a polyester made from dicarboxylic acid monomers (a carboxylic with a -COOH group at either end) and diols (an alcohol with an -OH group at either end)
Each -COOH group reacts with another -OH group on another monomer
An ester linkage is formed with the subsequent loss of onewater molecule per link
For every ester linkage formed in condensation polymerisation, one molecule of water is formed from the combination of a proton (H+) and a hydroxyl ion (OH–)
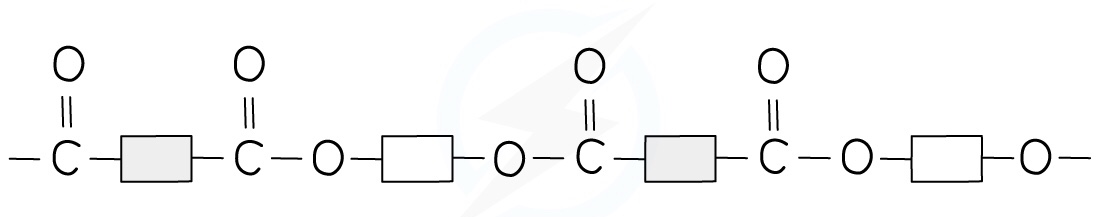
The structure of terylene can be represented by drawing out the polymer using boxes to represent the carbon chains
This can be done for all polyesters
18.3 Natural Polymers
Proteins and Carbohydrates
These are two of the main and most important components of food
Carbohydrates provide energy which is released during cellular respiration
Proteins are the building blocks of cells and are essential for growth
Biological catalysts also consist of protein

Protein as Polymer
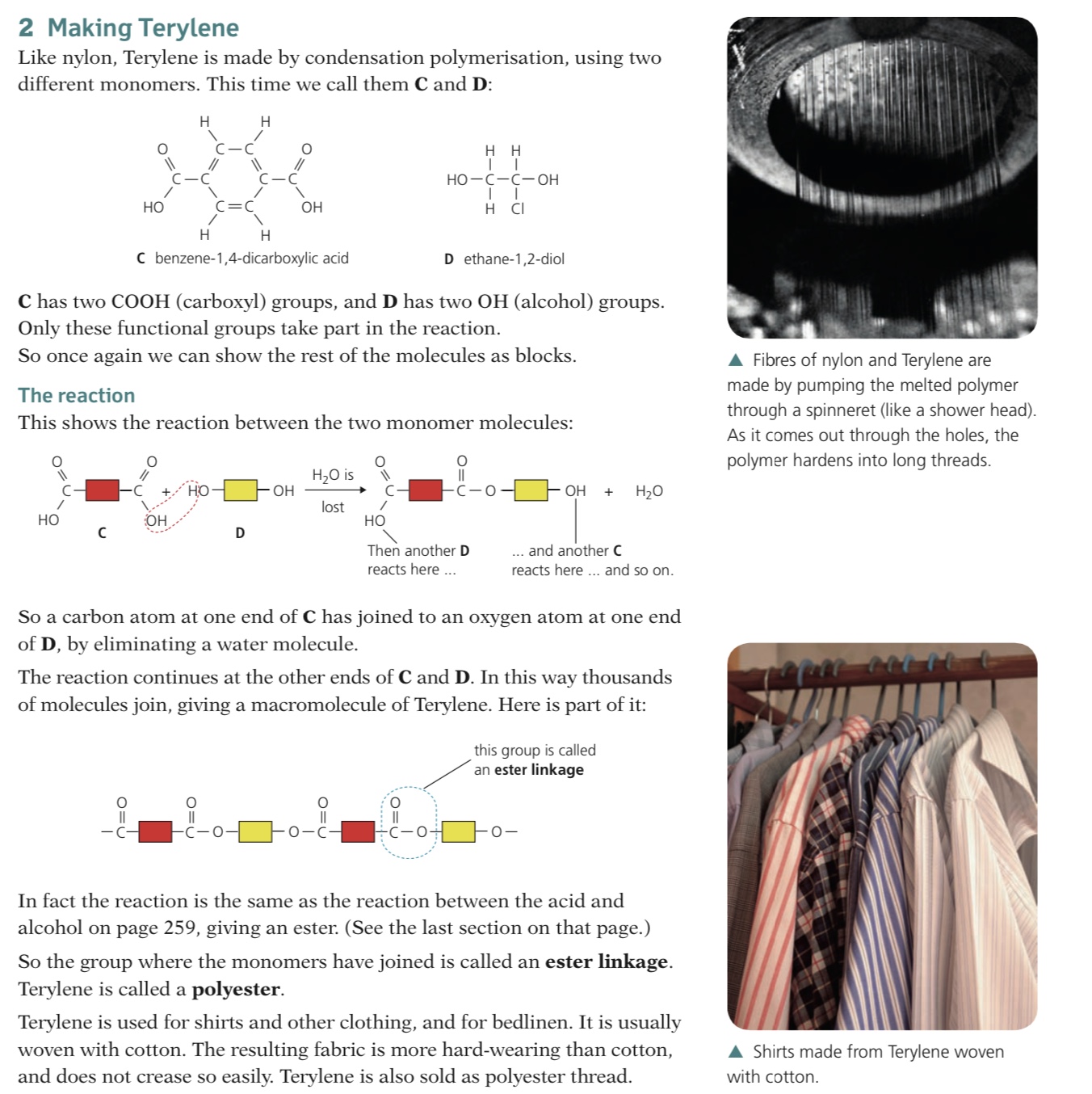
Proteins are condensation polymers which are formed from amino acid monomers joined together by amide links (in proteins also known as a peptide link) similar to the structure in nylon
The units in proteins are different however, consisting of amino acids
Amino acids are small molecules containing NH2 and COOH functional groups
There are twenty common amino acids, each differing by their side chain, represented by R
Proteins can contain between 60 and 600 of these amino acids in different orders
These are the monomers which polymerise to form the protein
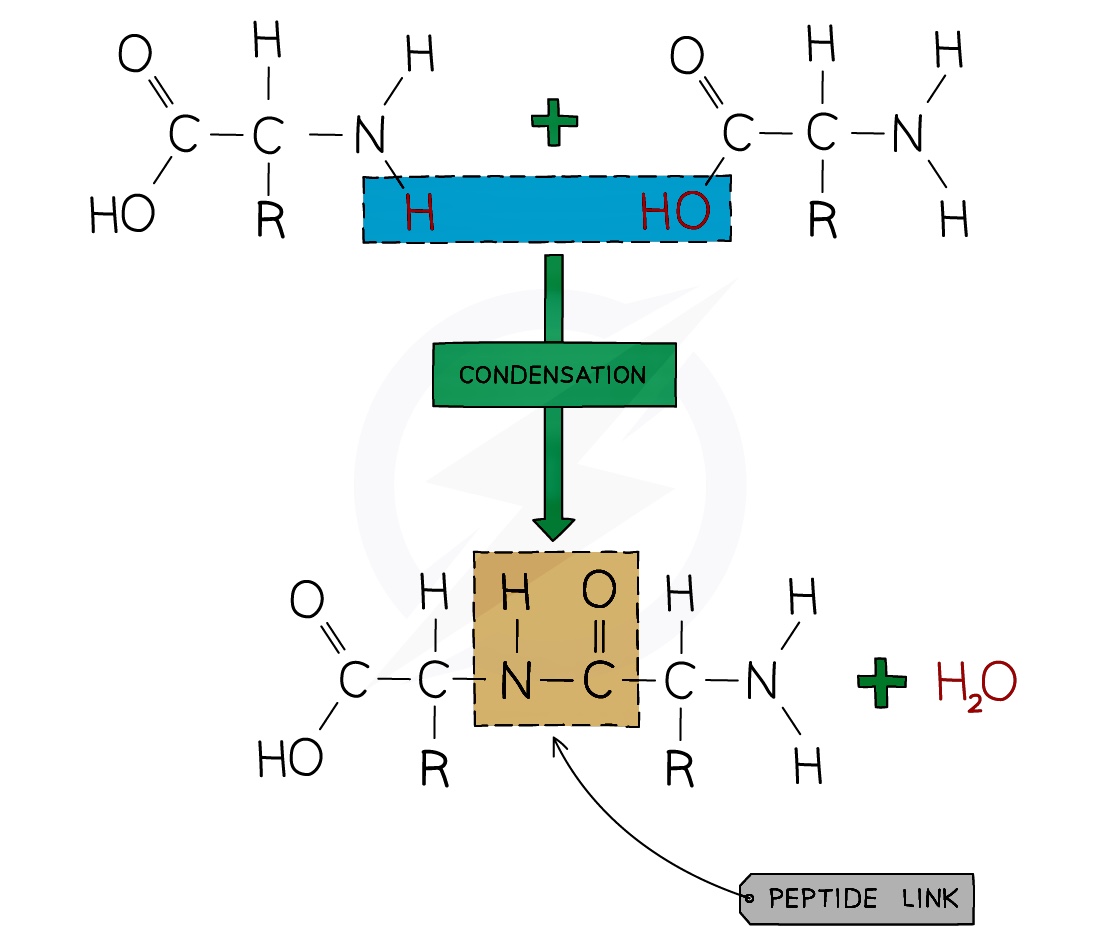
The structure of the protein can be represented using the following diagram whereby the boxes represent the carbon chains
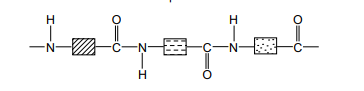
Hydrolysis of Protein
Hydrolysis is the splitting up of a molecule using water
When polymers are hydrolysed they will produce their monomers
Proteins can therefore be hydrolysed and will produce the monomer they were formed from, amino acids
In a lab this is done by heating them with concentrated hydrochloric acid
Carbohydrates, Fermentation and Chromatography
Carbohydrates are compounds of carbon, hydrogen and oxygen
There are simple carbohydrates and complexcarbohydrates
Simple carbohydrates are called monosaccharides and are sugars
- Examples include fructose and glucose
Complex carbohydrates are called polysaccharides
- Examples include starch and cellulose.
- They are usually made up of the same monomers, unlike proteins
- They are condensation polymers formed from simple sugar monomers
- A water molecule is eliminated due to it being a condensation reaction
- The linkage formed within the polymer is an -O- linkage called a glycosidic linkage
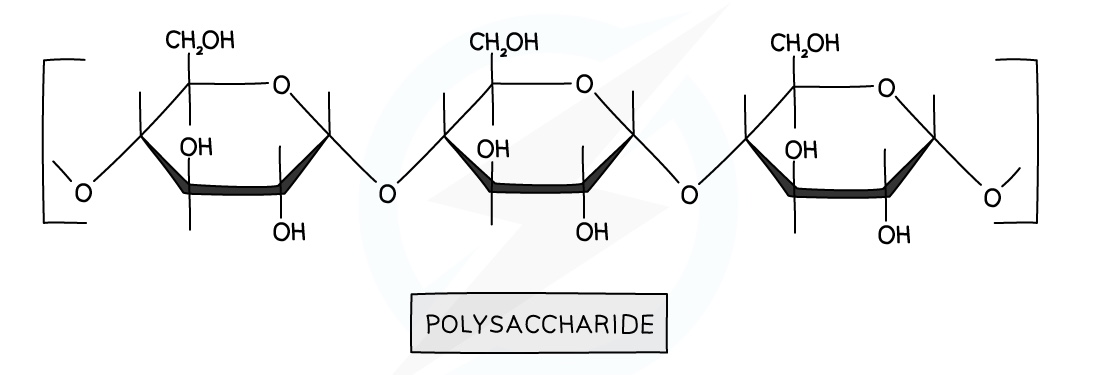
Hydrolysis of Carbohydrates
- The complex carbohydrates also undergo hydrolysis (water is used to split up the molecule) and produce the simple sugar monomers from which they were made
- In a lab this can be done by either:
- heating the carbohydrate with an acid, usually dilute HCl
- using enzymes
- In your body starch will produce glucose which can be then be used for respiration to produce energy
Fermentation of Simple sugars
- Simple sugars can be fermented to produce alcohol
- They are dissolved in water and yeast is added to be fermented between 15 and 35 °C in anaerobic conditions (absence of oxygen) for a few days
- If the temperature is too low the reaction rate will be too slow and if it is too high the enzymes will become denatured
- Yeast contains a naturally occurring enzyme, zymase (a biological catalyst) that breaks down starch or sugar to glucose
- The yeast respires anaerobically using the glucose to form ethanol and carbon dioxide:
C6H12O6 → 2CO2 + 2C2H5OH
Chromatography
The identification of the products of the hydrolysis of carbohydrates and proteins can be done using chromatography
Originally used for separating coloured substances, chromatography can also be used to identify colourless compounds, such as amino acids and simple sugars, using locating agents
Chromatography is carried out in much the same way, the only difference being that it is not obvious on the resulting chromatogram where the amino acids and sugars are located as they are colourless
The chromatogram is dried and sprayed with a locating agent to enable the substances to be seen
The Rf value can then be calculated for each dot and the sugar/amino acid identified
\n \n
\n \n