Unit 6
Two factors determine fate of chemical reaction: Direction and Rate
6.1 Energy comes in different forms
Kinetic Energy: energy associated with movement
Potential Energy: energy that a substance/object possess due to its structure and location
Types of energy used in bio
Light: Form of electromagnetic radiation visible to the eye, composed of photons
Heat: transfer of kinetic energy from one object to another
Mechanical: energy from an object due to its motion
Chemical Potential: potential energy stored between chemical bonds
Electron/Ion Gradient: the movement of charges creates energy and electrochemical gradient
Thermodynamics
First law: Energy cannot be created or destroyed, only transferred or transformed
Second law: Energy transfers/transformation increases disorder the the system, called entropy
Change in free energy determines direction of chemical reaction
Enthalpy (H) = total energy
Free Energy (G) = Useable energy
System’s Entropy (S) = unusable energy
T = temperature in Kelvin
Scientists use the change in free energy (delta G) to determine the direction of the reaction
formula: delta G = delta H - (T)delta S
If delta G < 0, reaction is exergonic, so energy is released
this is spontaneous
If delta G > 0, reaction is endergonic, so energy is used to start reaction
this favors formation of reactants
6.2 Enzymes
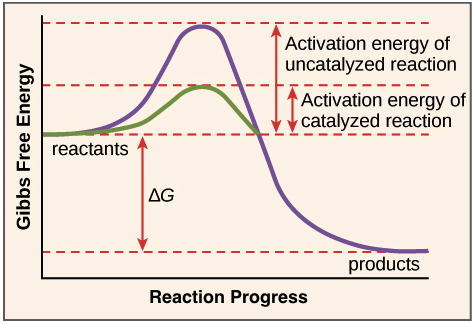
Enzymes catalyzed reactions occur at higher & faster rates
Activation energy: initial input of energy needed for rearrangement of bonds
Transition state: when original bonds stretch to create new products
Activation energy is a barrier to the formation of new products
Enzymes can lower the activation energy needed
Enzymes do this via 2 methods
Straining reactants: large enzyme proteins bind to smaller reactants, which strains the bonds of reactants, making it easier for them to reach transition state
Positioning reactants close together: Involves 2 or more reactants, enzymes can provide sites (active site) where reactants are close together
Enzymes recognize their substrate
Active site: where the chemical reaction takes place on an enzyme
Substrate: the reactant molecules that bind to active site
process is called enzyme-substrate complex

Additional factors influence enzyme function
enzymes sometimes require help to perform functions
Prosthetic Groups: small molecules that are permanently bonded to the surface of the enzyme, aids in enzyme function
Cofactors: usually inorganic ions that temporarily bond to surface of an enzyme, promotes chemical reactions
Coenzymes: organic molecules that temporarily bond to an enzyme
Ability of enzymes are affected by temp, pH, and ionic conditions
6.3 Metabolic Pathways
Series of steps if chemical reactions
each step is catalyzed by different enzymes
Catabolic Enzymes: The breakdown of larger molecules to smaller ones
Anabolic reactions: Smaller molecules are synthesized into larger ones
Catalytic reactions recycle organic building blocks
Breaking down larger molecules lets the body recycle the building blocks for other molecules
This can also release energy as the bonds of certain molecules contain a lot of chemical potential energy
ATP → ADP + Phosphate
Redox reactions transfer electrons
Oxidation: the removal of electrons during the breakdown of small molecules
oxygen is often involved in this process
Reduction: the addition of one or more electrons to an atom or molecules
Metabolic pathways are regulated in 3 general ways
Gene Regulation: enzymes are proteins coded by genes
These genes can be turned off/on
Cell-Signaling Pathways: cells react to signals from their environment and adjust their metabolic pathways to adapt
Biochemical Regulation: noncovalent bonding of a molecule to an enzyme
called Feedback Inhibition
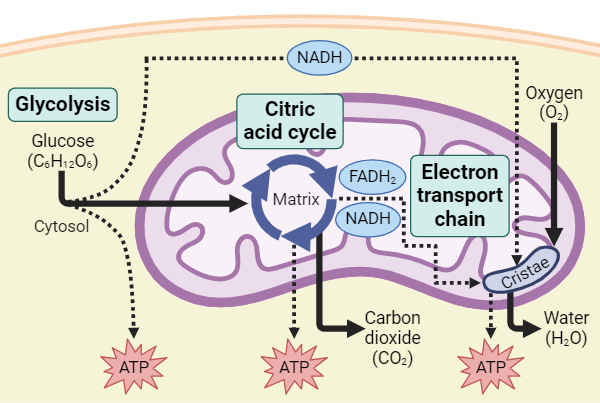
6.4 Overview of Cellular Respiration
The process of metabolic reactions that a cell uses to get energy from organic molecules and release waste products
Occurs in Four stages
Glycolysis
Glucose is broken down into 2 Pyruvate
occurs in cytosol
Creates 2 NADH and 4 ATP
Net total is 2 ATP
Breakdown of Pyruvate
2 Pyruvate is broken into 2 acetyl groups, which then bind to Coenzyme A (CoA)
Occurs in Mitochondrial Matrix
Creates one CO2 molecule
Citric Acid Cycle
2 acetyl groups are incorporated into organic molecule
Creates 4 CO2, 2 ATP, 6 NADH, and 2 FADH2
Oxidative Phosphorylation
NADH & FADH contain high energy electrons that are transferred in a redox reaction to other molecules
when removed, creates H+ electrochemical gradient
Mainly the process of electron transport and ATP synthesis
This occurs in the Cristae of the mitochondria
6.5 Glycolysis
Occurs in 3 main phases
Energy Investment Phase: 2 ATP is hydrolyzed into ADP and 2 phosphate groups
these phosphate groups are then attached to glucose
This helps make later reactions exergonic
Cleavage
Glucose is broken down into 2 pyruvate
Energy liberation
produces 4 ATP, 2 NADH, and 2 Pyruvate

6.6 Breakdown of Pyruvate
Pyruvate is oxidized by enzyme pyruvate hydrogenase
CO2 is removed and remaining acetyl groups are bonded to CoA
The removed electrons are transferred to NAD+, creating 2 NADH
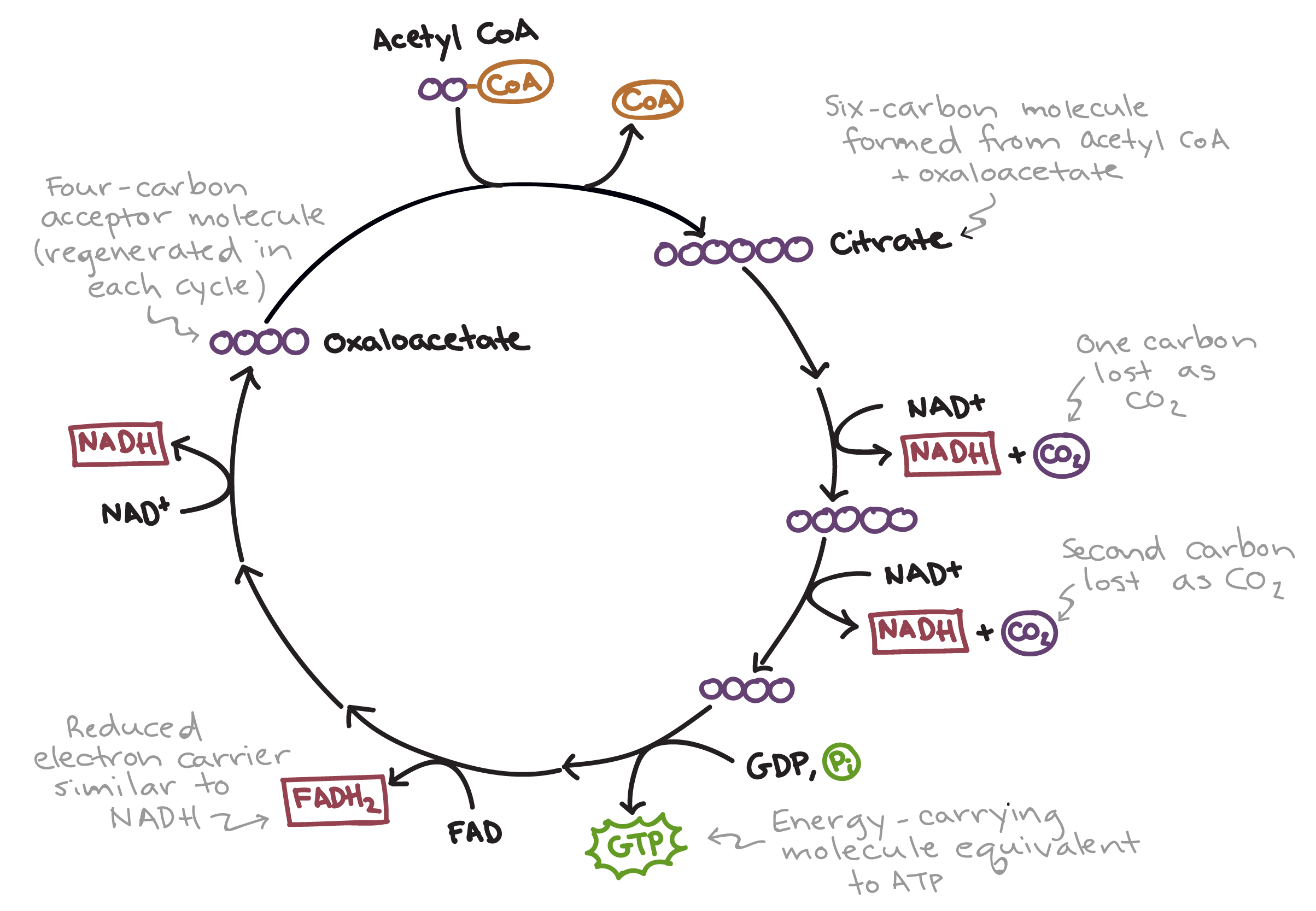
6.7 Citric Acid Cycle
Cyclical metabolic cycle
Acetyl CoA is converted into 4 CO2, 6 NADH, 2 ATP, and 2 FADH2
Regulated by the availability of substrates
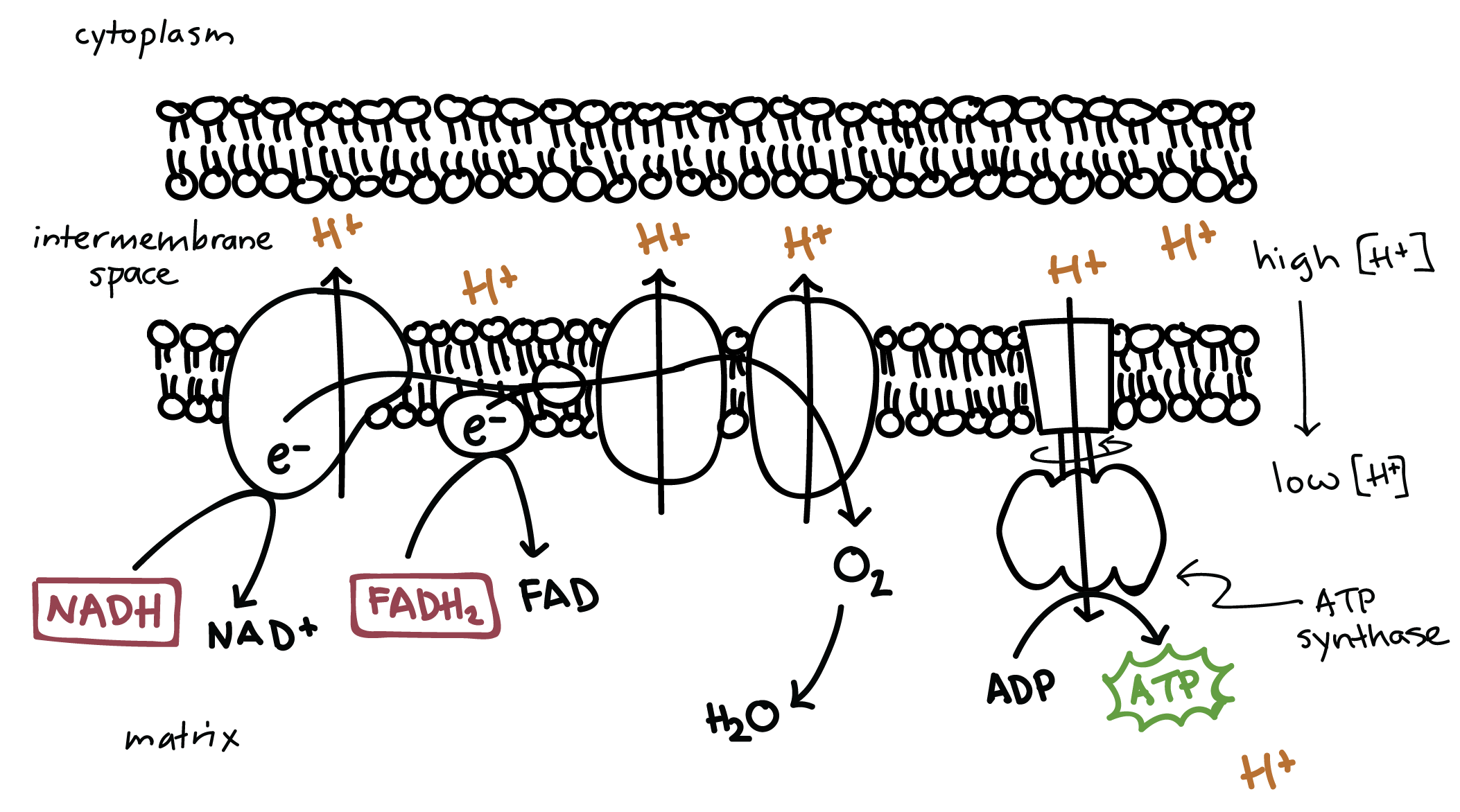
6.8 Oxidative Phosphorylation
Electron Transport Chain creates electrochemical gradient
consists of protein complexes inside mitochondrial membrane
As ETC moves through complexes, pumps H+ across membrane, creating an electrochemical gradient
ATP synthase makes ATP
ATP is synthesized by ATP Synthase
H+ electrochemical gradient is passive energy, aiding in the synthesis of ATP
H+ passes through ATP synthase, causing the top to spin, which creates ATP
Around 30 - 34 molecules of ATP are produced per cycle
6.9 Not really important
Proteins and fats can enter glycolysis and citric acid cycle at different times
6.10 Anaerobic respiration and fermentation
anaerobic: without oxygen
In an environment with little oxygen, cells use 2 ways to remedy it
Anaerobic Respiration
Some species evolved enzymes that trigger oxidative phosphorylation without oxygen
Fermentation
Molecules can make ATP through glycolysis in anaerobic environments
in anaerobic environments, citric acid cycle or ETC isn’t needed to make ATP
Glycolysis requires NAD+ to create NADH
in anaerobic environments, NADH increases, while NAD+ decreases
this causes NADH to haphazardly give away volatile electrons, damaging DNA and proteins
A decrease in NAD+ doesn’t maintain glycolysis
In muscle cells, lactate is secreted from pyruvate, which can maintain glycolysis until O2 is found