Chapter 9 - Solutions
9.1 - Solutions
- When a solvent dissolves in a solvent, a solution forms.
- The solvent particles are evenly dispersed into the solvent in a solution.
- Soluble, liquid, or gas can be the solution and solvent.
- Polar O-H bond leads to water molecular hydrogen bonding
- A polar solvent is an ionic solvent dissolved in water because the molecules of polar water attract and hydrate the ions into the solution.
- The word "dissolves" means that a polar or ionic solvent disintegrates into a polar solvent while a nonpolar solvent dissolves into a nonpolar solvent.
9.2 - Electrolytes and Nonelectrolytes
- Substances that create water ions, as their solutions conduct electrical power, are called electrolytes.
- Strong electrolytes are completely dissociated, but only partially disconnected weak electrolytes.
- Nonelectrolytes are substances that only produce molecules in water and cannot conduct electric streams.
9.3 - Solubility
A solvent has a solubility that can dissolve into 100 g of solvent as much as possible.
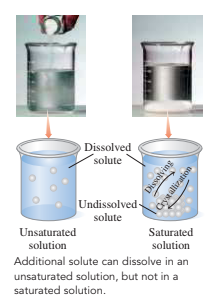
A saturated solution is a solution that includes the maximum dissolved solvent.
- The unsaturated solution contains less than the maximum dissolved solution.
An increase in temperature increases the solubility of most water solids but reduces the water solubility of gases.
- Soluble in water ionic compounds usually include Li+, Na+, K+, NH4+, NO3 -, C2H3O2-.
9.4 - Solution Concentrations
- Mass percent is the mass/mass ratio of solution mass to solution mass multiplied by 100%.
- The volume/volume (v/v) and mass/volume (m/v) ratios may also be the per cent concentration ratio.
- Molarity is the solute moles per liter solution.
- Concentration is used as a conversion factor for calculations of grams or milliliters of solution or solution
- Molarity (or moles/L) for the solution moles of solvent volume is written as a conversion factor.
9.5 - Dilution of Solutions
- A solution is added in the course of dilution, a solvent like water, which increases its volume and reduces its concentration.
9.6 - Properties of Solutions
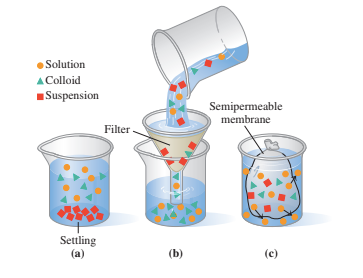
Colloids contain particles that are mostly filtered but not semipermeable membranes or settled.
- There are very large particles settling in suspensions.
The osmotic pressure is increased by the particles in a solution.
Solvent (water) transmissions from a lower osmotic (lower solution concentration) solution to a higher-osmotic (higher-osmotic) solution in osmotic conditions through a semi-permeable membrane.
Isotonic solutions are equal to body fluid osmotic pressures.
A red blood cell keeps its volume in an inside solution but shrinks into a hypotonic solution.
- In dialysis, a dialyzing membrane passes via water and small solvents while retaining larger particles.