Unit 2B: Thermodynamics
Internal Energy
the sum of all the molecular kinetic and potential energies
can change by adding heat or doing work
w = fdcosθ
Work in thermodynamics
consider the volume of a gas in thermodynamics equilibrium:
piston does work on the gas
w = F(-Δx) = PA(-Δx)
work done on the gas by external forces
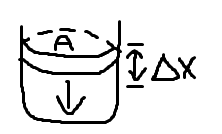
Change in volume: W = P(-ΔV)
if a gas expands, ΔV is positive and work done is negative because energy is lost
if a gas compresses, ΔV is negative and work is positive because energy is gained
W = -Wenv
Wenv is the work done by the gas
Example: a gas in a cylinder is at pressure 1.01×10^5 Pa and the piston has an area 0.100 m². as energy is slowly added to the gas by heat, the piston is pushed 0.04m. Calculate the work done by expanding the gas on its surroundings, Wenv.
W = -PΔV = -(1.01×10^5)(0.100-0.04) = -404 J
PV Graph

area = work = -PΔV = -P(Vf-Vi)
compression: volume shifts left
expansion: volume shifts right
First Law of Thermodynamics
energy conservation in relation to changes in internal energy, U, due to heat/work
changes in state— internal energy: P, T, V
ΔU = Uf - Ui = Q + W
Q is energy transfer
+Q = heat absorbed
-Q = heat released
W is work done
+W = work done on the environment (by the gas)
-W = work done on the system
Internal Energy of Monatomic Ideal Gas
ΔU = 3/2NKBΔT = 3/2nRΔT
Cv = 3/2R
Cv is the molar-specific heat at constant volume (isovolumetric)
ΔU = nCvΔT
Diatomic: Cv = 5/2R
4 Basic Types of Thermal Processes
Isobaric - constant pressure
Isovolumetric - constant volume
Isothermal - constant temperature
Adiabatic - no energy transferred by heat
Isobaric Process
ΔU = 3/2NKBΔT = 3/2nRΔT = 3/2PΔV
Q = ΔU - W = 3/2nRΔT + (+PΔV) = 5/2nRΔT
Cp = constant pressure = 5/2R
W = -PΔV
Isovolumetric Process
ΔU = 3/2NKBΔT = 3/2nRΔT = 3/2ΔPV
Q = CU = nCvΔT
Cv = 3/2R
W = 0
Isothermal Process
ΔU = 0
Q = - W
W = -nRTln(Vf/Vi)
Adiabatic Process
ΔU = W
Q = 0
W = ΔU
`P(V)^γ = const.
γ = Cp/Cv = adiabatic index
monatomic: 5/3
diatomic: 7/3
Cyclic Process
a system goes through a series of processes to return to the same initial state
internal energy = 0
ΔU = 0
total/net work done in a cyclic process equals the area enclosed in a PV diagram
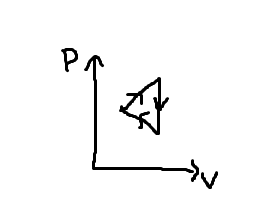
clockwise cycles = -w done on the gas (compression)
counterclockwise cycles = +w done on gas (expansion)
heat engines
takes in energy by heat and converts it to other forms of energy
work done by the eng = Weng
energy is expelled by the engine by heat to a source at a lower temperature
Q = -W
|Weng| = |Qh| - |Qc|
Qh = heat absorbed
Qc = heat lost
work done by heat engine = area
Thermal Efficiency of Heat Engine
e = Weng/|Qh| = 1 - |Qh|/|Qc|
Second Law of Thermodynamics
clausius statement: heat flows naturally from a hot object to a cold object but it will not flow spontaneously from a cold object to a hot object
kelvin-Planck statement: no process is possible in which the sole result is to transform a given amount of heat completely into work
e < 1
Some engine is always lost to a cold re4servoir
can’t break even
reversible processes are an idealization: every state along the path is an equilibrium state, so the system can return to its initial conditions by following this path in reverse
Irreversible processes: not possible— most real natural processes
if a real process occurs slowly enough, it can be considered to be almost reversible
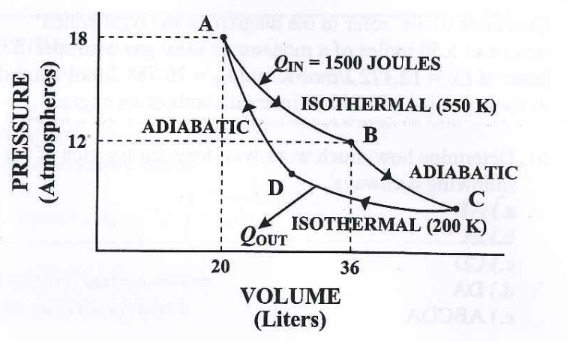
Carnot Engine/Cycle
most efficient → ideal reversible cycle
ideal gas contained in a cylinder with a moveable piston at one end
the temperature of a gas varies from Th to Tc
2 adiabatic and 2 isothermal reversible processes
Carnot’s Theorem: no real engine operating between two energy reservoirs can be more efficient than a Carnot engine operating
Tc/Th = Qc/Qh
Thermal efficiency: ec = (Th - Tc)/Th = 1 - (Tc/Th)
3rd Law of Thermodynamics
ec can only be 1 if Tc = 0k
Nernsts Theorem: It is impossible to decrease the temperature of a system tro absolute 0 in a finite number of operations
highest multiplicity = highest entropy = highest disorder
Disorder or Multiplicity
large amount of chance in natural processes
disorderly/random arrangements of objects are more probable than orderly ones
isolated systems tend toward greater disorder and entropy is a measure
greater probability/multiplicity = more entropy
entropy is a measure of multiplicity
Entropy in second law: cyclic processes → increase/remain the same
ΔS = Qr/T [J/k]
ΔS: change in entropy during any constant temperature between 2 states
Qr: energy absorbed/expelled during a reversible, constant temperature process
the entropy of the universe increases in all natural processes
total entropy of a system and environment increases
decreases: gain heat for one object, but will increase for another
entropy in a reversible adiabatic process: ΔS = 0
defines the direction of time
energy available for work decreases → leads to heat death