Radiation
Definition
Radiation is a form of energy that travels through space and can take the form of electromagnetic waves or particles.
It is categorized into two main types: ionizing radiation, which has enough energy to remove tightly bound electrons from atoms,
and non-ionizing radiation, which does not have sufficient energy for this process.
Why does Nuclear Decay occur?
Nuclear decay occurs because an atomic nucleus is unstable and seeks a more stable configuration.
The instability arises due to an imbalance between the forces that hold the nucleus together, particularly the strong nuclear force and the electrostatic repulsion between protons.
The 3 Types of Decay
Alpha (α) radiation
Fast moving particles, helium nuclei, ejected from certain radioactive nuclei.
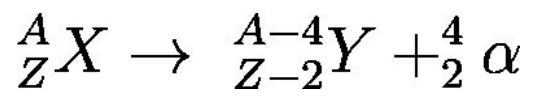
In heavy elements, the nucleus is too large for the strong nuclear force to counteract the electrostatic repulsion between protons.
Beta (β) radiation
Electrons with speeds just less than the speed of light, ejected from certain radioactive nuclei.
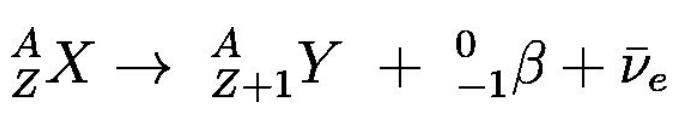
Imbalance in the neutron-to-proton ratio:
Too many neutrons → Beta-minus (β− beta) decay occurs to convert a neutron into a proton.
Too few neutrons → Beta-plus (β+ beta) decay occurs to convert a proton into a neutron.
Gamma (γ) radiation
Photons of high energy (high frequency, short wavelength) ejected from radioactive nuclei.
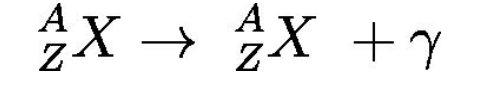
Excess energy in the nucleus:
After alpha or beta decay, the nucleus is sometimes left in an excited state (high energy). To reach a lower-energy, more stable state, the nucleus emits this extra energy as a gamma photon (high-energy electromagnetic radiation)
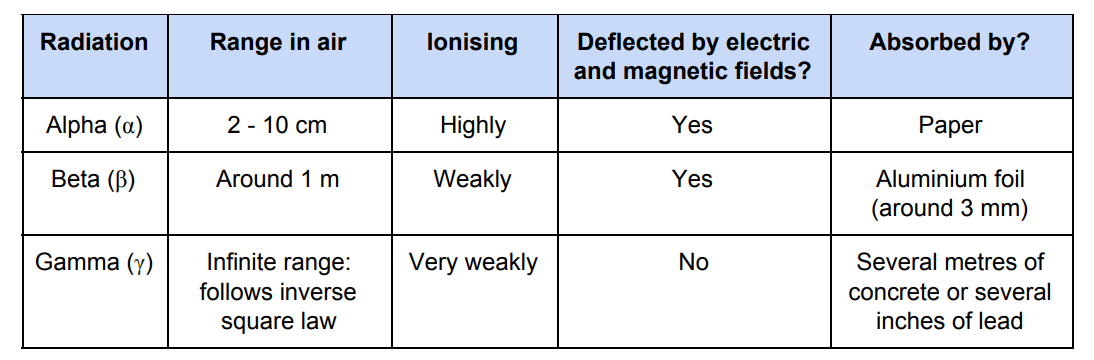
Table Summary of Properties