CHAPTER 22: CHEMISTRY OF THE NONMETALS
Periodic Trends and Chemical Reactions
- The chemistry exhibited by the first member of a nonmetal group can differ from that of subsequent members in important ways. Two differences are particularly notable:
- The first member is able to accommodate fewer bonded neighbors.
- The first member can more readily form p bonds and hence double and triple bonds.
Hydrogen
- [ ] Hydrogen — means “water producer,”; named by Antoine Lavoisier.
Isotopes of Hydrogen
[ ] Protium — The most common isotope of hydrogen, has a nucleus consisting of a single proton, which makes up 99.9844% of naturally occurring hydrogen.
[ ] Deuterium — makes up 0.0156% of naturally occurring hydrogen. It is not radioactive, and it is often given the symbol D in chemical formulas.
Deuteration — A process of replacing protium with deuterium, which can also have a profound effect on reaction rates, a phenomenon called a kinetic-isotope effect.
[ ] Tritium — A radioactive isotope of hydrogen with a mass approximately three times that of the common protium isotope.
Properties of Hydrogen
- Hydrogen is the only element that is not a member of any family in the periodic table.
- Hydrogen is sometimes placed above the halogens in the periodic table because the hydrogen atom can pick up one electron to form the hydride ion which has the same electron configuration as helium.
- Elemental hydrogen exists at room temperature as a colorless, odorless, tasteless gas composed of diatomic molecules.
- We can call H2 as dihydrogen, but it is more commonly referred to as either molecular hydrogen or simply hydrogen.
- Hydrogen forms strong covalent bonds with many other elements, including oxygen.
- When H2 is ignited in air, a vigorous reaction occurs, forming H2O. Air containing as little as 4% H2 by volume is potentially explosive. Combustion of hydrogen–oxygen mixtures is used in liquid-fuel rocket engines such as those of the Space Shuttle. The hydrogen and oxygen are stored at low temperatures in liquid form.
Uses of Hydrogen
- About half of the H2 produced is used to synthesize ammonia by the Haber process.
- Much of the remaining hydrogen is used to convert high-molecular-weight hydrocarbons from petroleum into lower molecular-weight hydrocarbons suitable for fuel (gasoline, diesel, and others) in a process known as cracking.
- Hydrogen is also used to manufacture methanol via the catalytic reaction of CO and H2 at high pressure and temperature.
Binary Hydrogen Compounds
- [ ] Ionic Hydrides — Are formed by the alkali metals and by the heavier alkaline earths (Ca, Sr, and Ba). These active metals are much less electronegative than hydrogen.
- [ ] Metallic Hydrides — Are formed when hydrogen reacts with transition metals. These compounds are so named because they retain their metallic properties.
- [ ] Molecular Hydrides — Are formed by nonmetals and metalloids, are either gases or liquids under standard conditions.
Group 8A: The Noble Gases
- The elements of group 8A are chemically unreactive.
- The group 8A elements are all gases at room temperature.
- They are components of Earth’s atmosphere, except for radon, which exists only as a short-lived radioisotope.
- Only Argon is relatively abundant.
- Neon, argon, krypton, and xenon are used in lighting, display, and laser applications in which the atoms are excited electrically and electrons that are in a higher energy state emit light as they return to the ground state.
- Argon is also used as a protective atmosphere to prevent oxidation in welding and certain high-temperature metallurgical processes.
- Liquid heliumis used as a coolant to conduct experiments at very low temperatures. \n
Noble-Gas Compounds
- The first noble-gas compound was reported in 1962. This discovery caused a sensation because it undercut the belief that noble-gas elements were inert.
- The initial study involved xenon in combination with fluorine, the element we would expect to be most reactive in pulling electron density from another atom.
Group 7A: The Halogens
The elements of group 7A, the halogens, have the outer-electron configuration ns^2 np^5, where n ranges from 2 through 6.
Chlorine, bromine, and iodine are found as the halides in seawater and in salt deposits.
Fluorine occurs in the minerals fluorspar, cryolite, and fluorapatite.
Only fluorspar is an important commercial source of fluorine.
Uses of the Halogens
- Fluorine is used to prepare fluorocarbons — very stable carbon–fluorine compounds used as refrigerants, lubricants, and plastics.
- Teflon — A polymeric fluorocarbon noted for its high thermal stability and lack of chemical reactivity.
- Chlorine is by far the most commercially important halogen. About half of the chlorine is used to manufacture chlorine-containing organic compounds, such as the vinyl chloride used in making PVC plastics.
- Sodium hypochlorite — The active ingredient in many liquid bleaches. Chlorine is also used in water treatment to oxidize and thereby destroy bacteria.
- Iodine is commonly used as KI in table salt**.**
- Iodized salt provides the small amount of iodine necessary in our diets; it is essential for the formation of thyroxin, a hormone secreted by the thyroid gland.
- Goiter — Lack of iodine in the diet results in an enlarged thyroid gland.
Hydrogen Halides
- The hydrogen halides form hydrohalic acid solutions when dissolved in water. These solutions have the characteristic properties of acids, such as reactions with active metals to produce hydrogen gas.
Interhalogen Compounds
- These are compounds formed when halogen group elements react with each other.
Oxygen
- Oxygen is found in combination with other elements in a great variety of compounds such as water, silica, alumina, and the iron oxides.
Properties of Oxygen
- Oxygen has two allotropes, O2 and O3.
- At room temperature, dioxygen is a colorless and odorless gas. Dioxygen is only slightly soluble in water (0.04 g/L, or 0.001 M at 25 °C), but its presence in water is essential to marine life.
- Oxygen can complete its octet of valence electrons either by picking up two electrons to form the oxide ion or by sharing two electrons.
Uses of Oxygen
- Oxygen is by far the most widely used oxidizing agent in industry.
- Over half of the O2 produced is used in the steel industry, mainly to remove impurities from steel. It is also used to bleach pulp and paper.
- Oxygen is used together with acetylene in oxyacetylene welding.
Ozone
- A pale blue, poisonous gas with a sharp, irritating odor.
- It is s a stronger oxidizing agent than dioxygen.
- Ozone can be prepared by passing electricity through dry O2.
- Ozone is sometimes used to treat drinking water.
- It is an important component of the upper atmosphere, where it screens out ultraviolet radiation and so protects us from the effects of these high-energy rays.
Oxides
- Acidic anhydrides/oxides — Oxides that form acids when they react with water.
- Basic anhydrides/oxides — Ionic oxides that dissolve in water form hydroxides.
Peroxides and Superoxides
- Peroxides — Compounds containing O—O bonds and oxygen in the –1 oxidation state.
- Super Oxide — A compound that contains the superoxide ion, which has the chemical formula O−.
- Disproportionation — An element is simultaneously oxidized and reduced.
- Hydrogen peroxide is marketed as a chemical reagent in aqueous solutions of up to about 30% by mass.
- The peroxide ion is a by-product of metabolism that results from the reduction of O2. The body disposes of this reactive ion with enzymes such as peroxidase and catalase. \n
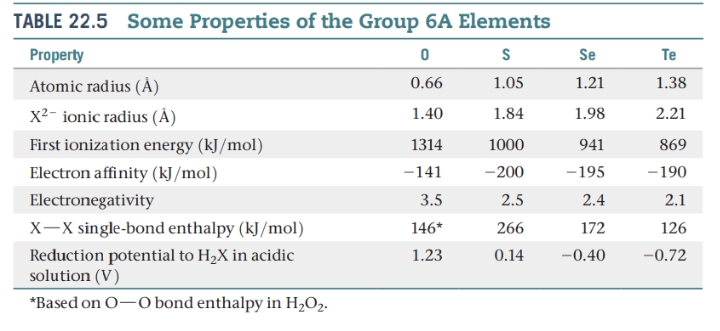
Other Group 6A: S, Se, Te, and Po
The other group 6A elements are sulfur, selenium, tellurium, and polonium. Of these, sulfur is the most important, and polonium is the least important.
\n Properties and Uses of Sulfur and Selenium
- Elemental sulfur is yellow, tasteless, and nearly odorless. It is insoluble in water and exists in several allotropic forms. The thermodynamically stable form at room temperature is rhombic sulfur, which consists of puckered S8 rings with each sulfur atom forming two bonds.
- Most of the sulfur produced in the United States each year is used to manufacture sulfuric acid. Sulfur is also used to vulcanize rubber, a process that toughens rubber by introducing cross-linking between polymer chains.
- Selenium is used in photoelectric cells and light meters because its electrical conductivity increases greatly upon exposure to light.
- Photocopy machines contain a belt or drum coated with a film of selenium. This drum is electrostatically charged and then exposed to light reflected from the image being photocopied.
Nitrogen
- Nitrogen constitutes 78% by volume of Earth’s atmosphere, where it occurs as N2 molecules.
- It is a key element in living organisms, compounds of nitrogen are not abundant in Earth’s crust.
Properties of Nitrogen
- Nitrogen is a colorless, odorless, tasteless gas composed of N2 molecules.
- The N2 molecule is very unreactive because of the strong triple bond between nitrogen atoms. When substances burn in air, they normally react with O2 but not with N2.
Hydrogen Compounds of Nitrogen
- Ammonia — A colorless, toxic gas that has a characteristic irritating odor; one of the most important compounds of nitrogen.
- Hydrazine — Another important hydride of nitrogen. The hydrazine molecule contains an N—N single bond.
- Pure hydrazine is a strong and versatile reducing agent. The major use of hydrazine and compounds related to it, such as methylhydrazine
Oxides and Oxyacids of Nitrogen
- Nitrous oxide — Also known as laughing gas because a person becomes giddy after inhaling a small amount. This colorless gas was the first substance used as a general anesthetic. It is used as the compressed gas propellant in several aerosols and foams, such as in whipped cream.
- Nitric oxide — Also a colorless gas but, unlike N2O, it is slightly toxic. It can be prepared in the laboratory by reduction of dilute nitric acid, using copper or iron as a reducing agent.
- Nitrogen dioxide — A yellow-brown gas. It is a major constituent of smog. It is poisonous and has a choking odor.
- The two common oxyacids of nitrogen are nitric acid and nitrous acid.
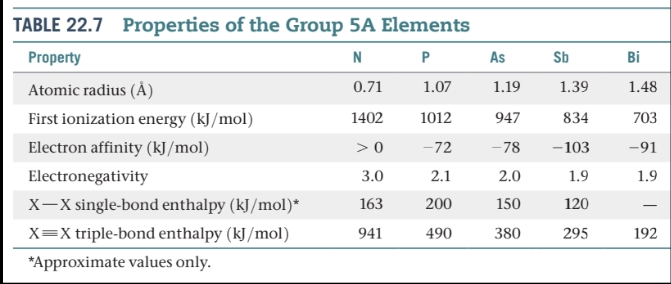
Other Group 5A: P, As, Sb, and Bi
Of the other group 5A elements — phosphorus, arsenic, antimony, and bismuth— phosphorus has a central role in several aspects of biochemistry and environmental chemistry.
\n Occurrence, Isolation, and Properties of Phosphorus
Phosphorus occurs mainly in the form of phosphate minerals. The principal source of phosphorus is phosphate rock, which contains phosphate principally as Ca3(PO4)2.
White phosphorus consists of P4 tetrahedra.
The bond angles in this molecule, 60°, are unusually small, so there is much strain in the bonding, which is consistent with the high reactivity of white phosphorus. This allotrope bursts spontaneously into flames if exposed to air.
Red phosphorus is also considerably less poisonous than the white form.
\n Phosphorus Halides
Phosphorus trichloride (PCl3) is commercially the most significant of these compounds and is used to prepare a wide variety of products, including soaps, detergents, plastics, and insecticides.
Phosphorus chlorides, bromides, and iodides can be made by direct oxidation of elemental phosphorus with the elemental halogen.
Carbon
- Carbon constitutes only 0.027% of Earth’s crust. Carbon is also found in coal, petroleum, and natural gas. The importance of the element stems in large part from its occurrence in all living organisms: Life as we know it is based on carbon compounds.
Elemental Forms of Carbon
- Graphite — A soft, black, slippery solid that has a metallic luster and conducts electricity; it consists of parallel sheets of sp^2 hybridized carbon atoms held together by dispersion forces.
- Carbon Black — Used as a pigment in black inks; large amounts are also used in making automobile tires.
- Charcoals — Formed when wood is heated strongly in the absence of air.
- Activated Charcoal — A pulverized form of charcoal whose surface is cleaned by heating with steam, is widely used to adsorb molecules.
- Diamond — A clear, hard solid in which the carbon atoms form an sp^3 hybridized covalent network. It is denser than graphite
Oxides of Carbon
- Carbon monoxide — Is formed when carbon or hydrocarbons are burned in a limited supply of oxygen.
- It is a colorless, odorless, tasteless gas that is toxic because it binds to hemoglobin in the blood and thus interferes with oxygen transport.
- Carbon monoxide is unusual in that it has a nonbonding pair of electrons on carbon
- Carbon dioxide — Is produced when carbon-containing substances are burned in excess oxygen.
- It is also produced when many carbonates are heated.
Carbonic Acid and Carbonates
- Carbonic acid — Is a weak diprotic acid. Its acidic character causes carbonated beverages to have a sharp, slightly acidic taste.
- Principal Carbonate Minerals: Calcite, Magnesite, Dolomite & Siderite.
- Calcite — Is the principal mineral in limestone and the main constituent of marble, chalk, pearls, coral reefs, and the shells of marine animals such as clams and oysters.
Carbides
- Carbides — Is the binary compounds of carbon with metals, metalloids, and certain nonmetals.
- The more active metals form ionic carbides, and the most common of these contain the acetylide ion.
- The most important ionic carbide is calcium carbide.
- It is a very strong base that reacts with water to form acetylene.
- Interstitial carbides are formed by many transition metals. The carbon atoms occupy open spaces (interstices) between the metal atoms in a manner analogous to the interstitial hydrides.
- Covalent carbides are formed by boron and silicon.
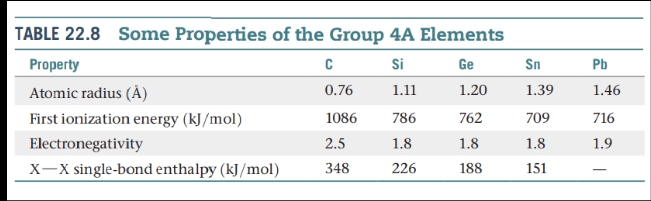
Other Group 4A: Si, Ge, Sn, and Pb
Carbon is a nonmetal; silicon and germanium are metalloids; tin and lead are metals.
\n Occurrence and Preparation of Silicon
Silicon is the second most abundant element, after oxygen, in Earth’s crust. It occurs in SiO2 and in an enormous variety of silicate minerals.
Elemental silicon has a diamond-like structure.
Crystalline silicon is a gray metalliclooking solid that melts at 1410 °C.
Zone Refining — Can help further purify the element.
- As a heated coil is passed slowly along a silicon rod, a narrow band of the element is melted.
- As the molten section is swept slowly along the length of the rod, the impurities concentrate in this section, following it to the end of the rod. \n
Silicate
- Silicate: Silicon atom is surrounded by four oxygens and silicon is found in its most common oxidation state, +4.
- Asbestos — Is a general term applied to a group of fibrous silicate minerals. The structure of these minerals is either chains of silicate tetrahedra or sheets formed into rolls.
Silicone
- Silicones consist of O—Si—O chains in which the two remaining bonding positions on each silicon are occupied by organic groups such as CH3.
- Silicones are nontoxic and have good stability toward heat, light, oxygen, and water.
- They are used commercially in a wide variety of products, including lubricants, car polishes, sealants, and gaskets.
- They are also used for waterproofing fabrics. When applied to a fabric, the oxygen atoms form hydrogen bonds with the molecules on the surface of the fabric.
Boron
Boron is the only group 3A element that can be considered nonmetallic.
[ ] Borane anions — Boron and hydrogen form a series of anions.
[ ] Boric Oxide — The only important oxide of boron.
[ ] Boron Hydrides — Boron forms a variety of compounds with hydrogen.
Boranes react with oxygen to form boric oxide (B2O3), in which boron is in the +3 oxidation state.
Boric oxide is the anhydride of boric acid (H3BO3). Boric acid readily undergoes condensation reactions.
\n