Rate of Reaction
There are 4 factors that affect the rate of a reaction:
Concentration of reactants (pressure of gases)
Particle size of reactants
Temperature
Presence of a catalyst
Collision Theory
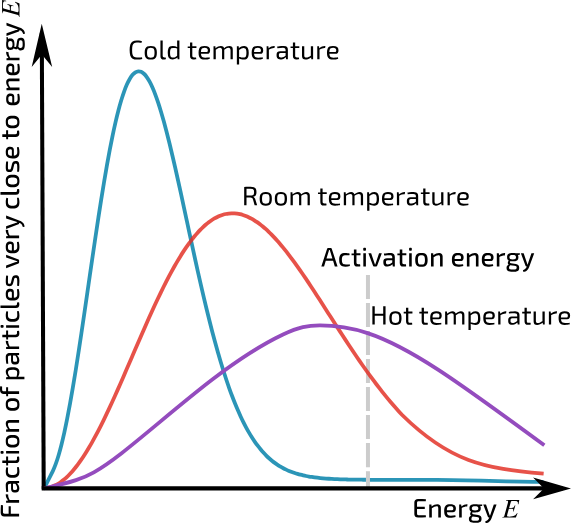
A reaction will only occur if the particles collide with sufficient energy (E > Ea)
Concentration of Reactants
Increasing concentration of reactants increases rate of reaction.
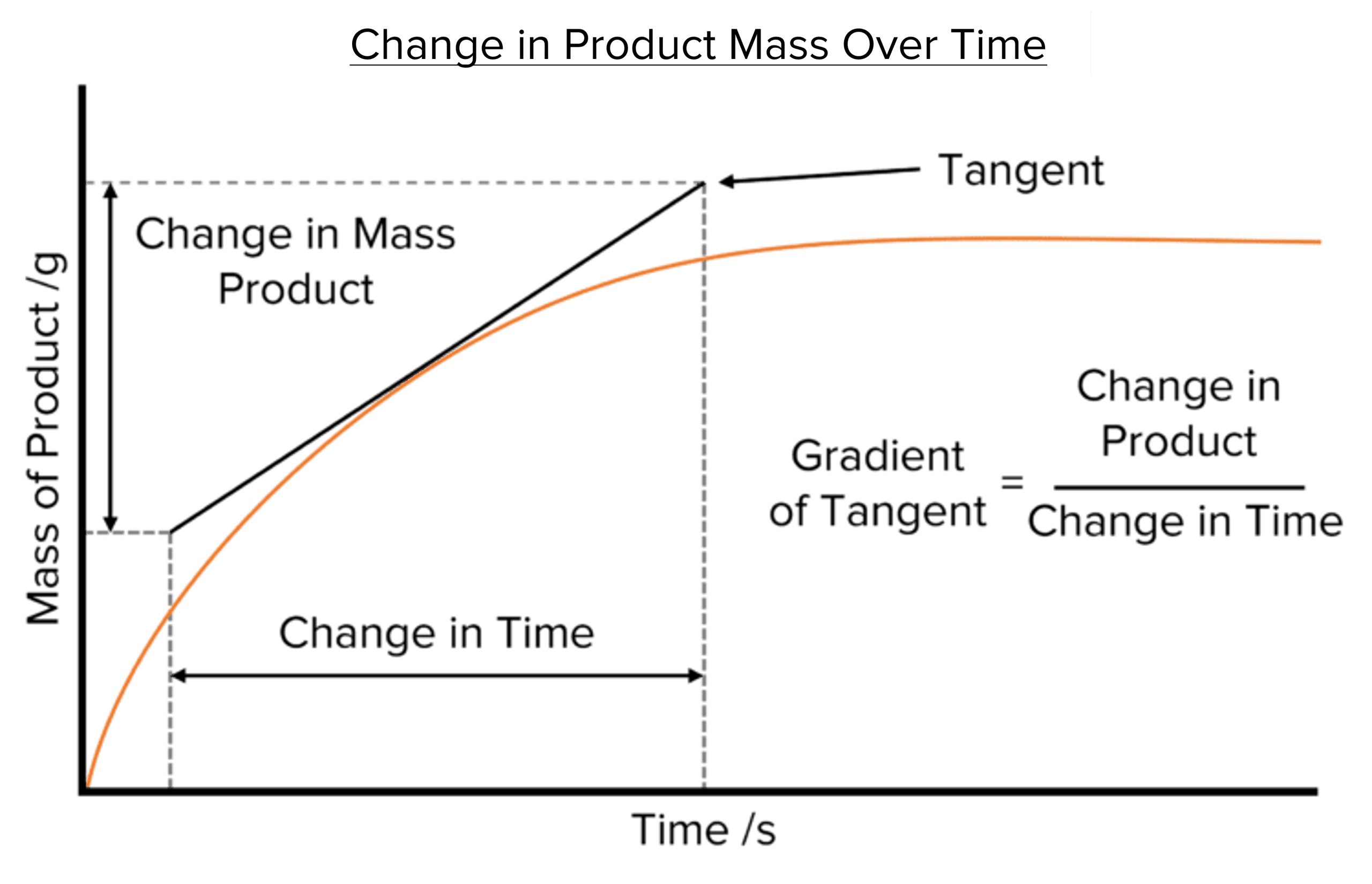
Reaction is greatest at the start (steepest slope); greatest concentration of reactant particles
Reaction slows down with time as HCl is being used up (limiting factor)
Reaction stops when gradient = 0; All HCl has reacted.
If there are twice as many reactant particles in the same volume: this will double the collision frequency and therefore double number of successful collisions— rate doubles
Average rate of reaction = total volume of product(s) formed / time taken to form in cm3s-1.
If the surface area of the reactants are doubled: double the number of reactant particles are exposed to the solution. This will double the collision frequency and double the number of successful collisions with energy greater than activation energy. Therefore this doubles the rate of reaction.
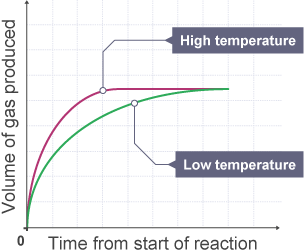
Effect of Temperature
Increase in temperature, means an increase in the mean kinetic energy of the particles. So they move faster, therefore their is a greater collision frequency. MANY MORE PARTICLES have energy greater than activation energy, and lead to a successful collision.
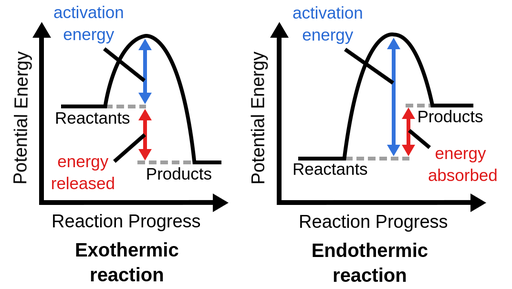
Endothermic Reactions
Energy taken in from surroundings; more chemical energy stored in products than reactants
Exothermic Reactions
Energy releases into surroundings; more chemical energy stored in reactants than products
MAXWELL-BOLTZMANN DISTRIBUTION
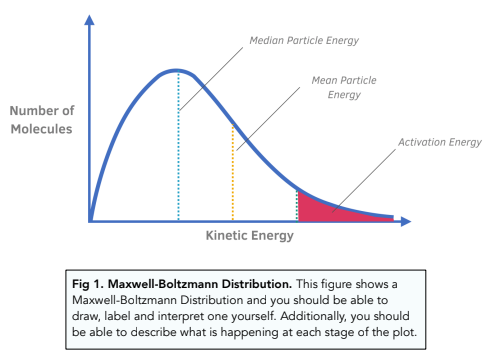
Catalysis
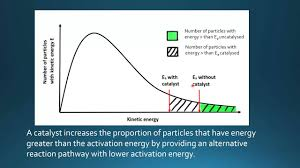
By providing an alternate pathway with a lower activation energy, without being used up, it increases the rate of reaction as more particles have energy > activation energy.
Activation Energy
Minimum amount of energy required to start a reaction.
 Note
Note Studied by 25 people
Studied by 25 people Note
Note Studied by 3 people
Studied by 3 people Note
Note Studied by 11 people
Studied by 11 people Note
Note Studied by 8 people
Studied by 8 people Note
Note Studied by 3 people
Studied by 3 people Note
Note Studied by 17 people
Studied by 17 people Knowt
Knowt




