Antibacterial agents
Antibacterial agents
Protein synthesis
30s subunit
Tetracyclines: Doxycycline (second generation)
Mechanism of Action
- Goes through the outer membrane via passive diffusion and active transport in G+
- Transverses through OmpF & OmpC porin channels in G -
- Then active transport through cell membrane
- Binds to the 30S subunit: Competes with the Aminoacyl-tRNA on the A site, and thereby prevents formation of the polypeptide chain
Pharmacokinetics:
- Administration: Orally or IV (only doxycycline in clinical setting)
- Absorption - administered with dairy products - can form nonabsorbable chelates with mg, Fe & Ai cations.
- Distribution - Everywhere. Undergoes calcification in teeth, bones and tumours with high Ca2+ content. Only doxycycline & minocycline cross BBB
- Metabolism - first generation not metabolized, 2nd generation partly metabolised in liver
- Excretion - by kidneys in urine, accumulates in renal failure. 2nd gen: excreted in bile.
Spectrum of activity
- Very wide, including G+ & G- bacteria, mycoplasma, rickettsiae, spirochaetes, protozoa
- Were taken preventatively and therefore there has become a lot of resistance
Clinical use
- Peptic ulcer disease
- Lymes disease (e.g. borellia)
- Mycoplasma pnemoniae
- Cholera
- Chlamydia
- etc…
Side effects
- GIT disturbances: nausea, bowel upset
- Deposition in calcified tissues: lead to discoloration and hyperplasia of teeth
- Photosensitivty: can cause sunburn
- Hepatotoxicity: rare, pregnenant women
- Vestibular disfunction: dizziness, vertigo & tinnitus
- Haematolofic toxicity
- Fanconi syndrom: electrolyte imbalance
- Pseudotumor cerebri: hypertension in brain - headaches & blurred vision
Resistance
- Big problem!
- Efflux pumps
- Enzymatic inactivation, a bit rarer
- Ribosomol protection: blocking the tetracyclines from binding, distorting structure or dislodging tetracycline.
Aminoglycosides: Streptomycin
Mechanism of Action
- Bactericidal
- The aminoglycosides are taken up through increased permeability of the bacterial membrane: They bind to negatively charged components of bacterial membrane. This will cause a displacement of Mg ions: which will destabilize the lipid components and thereby increase the permeability. However, they are probably taken up by porins as well. This is through the cell wall.
- To get past the cell membrane they use active transport. This is an oxygen-dependent transport and it is therefore not functional against anaerobes.
- It then binds to the A-site on the 30S and can block it in three ways:
- Block initiation complex
- Cause alteration in codon; anticodon recognition
- Promote mistranslation by inducing codon misreading on delivery of the aminoacyl tRNA
- This will allow more aminoglycosides in, because there will be channels that are introduced which are mistranslated proteins.
Pharmacokinetics:
- IV - it’s not absorbed in GIT
- Topical for local use
- Mainly to extracellular fluid
- Crosses placenta and into joints
- Renal excretion
Spectrum of activity
- Broad spectrum against + and -, including multidrug resistant bacteria
- Active against biothreats e.g. Yersinia pestis and francisella tularensis
- → No activity against anaerobic bacteria
Clinical use
- Used against gram negative, e.g. septicaemia (blood infections).
- Used for bacterial endocarditis (infection in heart tissues)
- Tuberculosis, plauge
Side effects
- Toxicity is a risk at high dosages
- Ototoxicity (causing hearing loss)
- Nephrotoxicity related to dosage
- Haematological toxicity
- Neuromusuclar transmission
- Allergic reaction
Resistance
- Decreased uptake/accumulation due to membrane impermeabilization. → can be overcome by using inhibitors of cell wall synthesis in the same dosage.
- Drug inactivating enzymes
- Deletion/alteration of receptor protein on 30S which prevents attachment.
50s subunit
Chloramphenicol
General facts
- First broad-specturm antibiotic discovered
- Chloromycetin in the US
- Bacteriostatic, but can be bactericidal at high concentrations
Mechanism of Action
- penetrates through facilitated diffusion
- Binds to the 50S subunit, causing a conformational change. This will slow the binding of the tRNA to the A-site, and inhibits transpeptidation process (movement of peptide chain).
- Obs - it competes in binding the ribosome with macrolides & lincosamide, so combination treatments have no benefits.
Pharmacokinetics:
- Administration - oral, IV or topical as ear & eye drops
- Activation - the oral & IV are prodrugs: they are activated by hydrolysis in small intestine (oral) or converted to active form in circulation (IV).
- Absorption - oral rapidly absorbed from GIT, peak blood conc. after 2hrs. IV, serum levels are dependent on the patients metabolism. Plasma T1/2 ~4hrs.
- Distribution - everywhere, 60% in blood because binds to plasma proteins, can accumulate in braintissue.
- Metabolism - by hepatic glucuronyl transferase into inactive metabolites in liver
- Excretion - renal tube and excrete in bile, ~10% of drug excreted unchanged
Spectrum of activity
- Salmonella
- Chlamydiae
- Rickettsiae
- Spirochetes
- Mycoplasma
Clinical use
- Last resort drug for serious & life-threatening infections because there’s high toxicity
- Systemic uses include typhoid fever, cholera, anaerobic infections etc.
Side effects
- Anemias, causes bone marrow depression
- Drug interactions, can inhibit some liver drugs and therefore prevent the metabolism of drugs
- Occular irritation and toxicity, can cause blurred vision
- Grey baby syndrome
- Gastrointestinal disturbances, e.g. nasuea, vomiting, diarreah
- CNS effects, e.g. headach, depression, confusion
Resistance
- Enzymatic inactivation through acetylation by chloramphenicol acetyltransferase
- Decreased permeability
- Presences / increased presence in efflux pumps: the bacteria pumps out the drug
- Ribosomal protection, due to modification of binding site
Macrolides - e.g. Erythromycin
Mechanism of Action
- Bactericidal or static depending on concentration and type of micro-organism
- Binds to the 50s subunit
- Blocks translocation of t-RNA with polypeptide change from A-site to P-site.
- Therefore inhibits the protein synthesis
Pharmacokinetics:
- Most often oral, taken with or without food depending on type. Erythomycin IV
- Extracellular fluid
- Accumulates in prostatic fluid & macrophages
- Undergoes hepatic metabolism
Spectrum of activity
- G+ cocci and bacilli, and some G- cocci and bacilli
- Erythromycin is effective against many of the same organisms as penicillin G; streptococcus, legionella etc.
Clinical use
- Whooping cough
- community-acquired pneumonia
Side effects
- GIT disturbances
- Ototoxicity
- Drug interactions
- jaundice
- QT prolongation…
Resistance
- Efflux systems
- Drug inactivation
- Modification of the ribosome 50S → lower affinity for macrolides.
Nucleic synthesis
Alteration of the base-pairing properties of the template: Acrtidites
General facts
- are intercalating agents: meaning that they produce mutations by getting in between adjacent bases in the DNA, and therefore distorting the 3D structure of the helix.
- Examples are proflavine and acriflavine
Mechanism of Action
- Intercalcates into the DNA
- This doubles the distance between the pairs, causing disruption in DNA synthesis
- Causes frameshift mutations and therefore prevents bacterial reproduction
Clinical use
- Used as antibacterial agent during WW2 against G+ bacteria
- Only as a surface disinfectant or treating superficial wounds nowadays.
Toxicity
- Super toxic - cannot be used systemically.
- It’s carcinogenic in animals, because it gets into your DNA and stays there.
Inhibition of either DNA or RNA polymerase: Actinomycin D
General facts
- Intercalcalating
Mechanism of Action
- Intercalates in the minor groove of double helix between guanine-cytosine. GC-rich regions are proliferation genes
- It interferes with movement of RNA polymerase along the gene, therefore preventing transcription and triggers apoptosis of the cell
Spectrum of activity
- High inhibitory effect on gram +, - and some fungi
Clinical use
- Not first line of treatment due to strong side effects
- Used in combination with surgery for treatment of Wilm’s tumour and other rare diseases
- Part of combination chemotherapy, because it kills all cells in your body :-)
Pharmacokinetics
- Administration: By I.V.
- Absorption: Poorly via GT, therefore IV
- Distribution - Fast into tissue, mostly in bone marrow & nucleated cells. No BBB, but crosses placenta. Is free floating in blood.
- Metabolism - minimally metabolized in liver
- Excretion - excreted via bile (50-90%) and urine
Side effects
- Irritating to tissues
- Gastrointestinal distress - abdominal pain, diarrhoea, nasua etc
- Hepatotoxicity - can cause liver injury
- Haematological toxicity - can cause bone marrow depression
- Carinogenicity - can cause cancer
- hypersensitivity
Direct effects on DNA iteself: Metronidazole
General facts
Is an alkylating agent: contains a chemical group that produces highly reactive carbonium ion intermediates. These carbonium ions react with nucleophilic substances in the cell - especially with electron donors. It forms covalent bonds with bases in the DNA. It therefore prevents replication.
- Sold under name: Flagyl
- Bactericidal
Mechanism of Action
- Diffuses across the cell membrane via passive diffusion
- Is activated through reduction by intracellular transport proteins. This only happens in anaerobic cells - it is therefore relatively safe for humans.
- The nitro group of the molecule binds to the DNA. This causes loss of helical DNA and strand breakage -- preventing synthesis.
Spectrum of activity
- Used for anaerobic infections
- Can be used for antiprotozoal
- There’s not much effect on human cells or aerobic cells
Clinical use
- Used against anaerobic cocci and bacilli infections, e.g. of wound abscess and combination therapy against helicobacter pylori.
- Used against anaerobic infections after bowel surgery
- Etc
Pharmacokinetics:
- Administration - Oral & IV
- Absorption - Rapidly absorbed after both
- Distribution - Oral bioaailability almost 100%! Goes everywhere, crosses BBB, <20% bound to plasma proteins
- Metabolism - Hepatic metabolism (30-60%)
- Excretion - Kidneys in urine, some fecal elimination
Side effects
- GIT distress; cramps & nausea
- Neurotoxicity: dizziness, vertigo, seizures, numbness
- Dermatological effects: Rashes & hives
- Steven-Johnson syndrome: rare flu-like syndromes with rashes, only found in combination with mebendazole
Drug interactions
- Acohol: causing nausea, vomiting, cramps
- Anticoagulants - prolonged prothrombin time
- Cimetidine - prologons the half life metronidazole
Resistance
- Rare, can be caused by specific resistance genes.
Inhibition of DNA gyrase: Fluoroquinolones
General facts
DNA gyrase is an essential bacterial enzyme that unwinds the helix. It’s a type of topoisomerase.
- Most used: Ciprofloxacin
- Bactericidal
Mechanism of Action
- Inhibits topoisomerases/DNA gyrases
- This will cause permanent gaps in the DNA strands. This will cause repair by exonucleases. This will lead to breakdown of DNA and irreversible damage → death of bacteria.
- In G negative: Topoisomerase II, that normally prevents supercoiling of the DNA. Here: There’s no effect on transcription or replication
- In G positive: Topoisomerase IV, that normally relaxes supercoiled DNA
Pharmacokinetics:
- Administration - Orally, IV and Topically
- Absorption - Well absorbed from GIT (80-90%). With IV, dietary supplements containing Fe, Zn or Ca interfere with absorption.
- Distribution - Widely distributed in all tissues. Plasma binding 10-40%, Penetration of BBB is low (except ofloxacin which crosses BBB well). Can accumulate in macrophages.
- Metabolism - Hepatic metabolism
- Excretion - primarily renal - renal failure can cause toxicity. Some bile excretion.
Spectrum of activity
- Against G- organisms
- E.g. mycobacteria & legionella pneumophila
- Less active against G+ due to resistance
Clinical use:
There is a lot of different types of fluoroquinolones that have different clinical uses:
- Nalidixic acid, norfloxacin: urinary tract infections
- Ciprofloxacin: main one used, mostly against G- bacteria, e.g. chlamydia.
- Levofloxacin: used against streptococcus pneumoniae e.g.
Side effects
- GIT: Diarrhoea
- CNS effects: Headache, dizziness, confusion
- Allergic reactions: rashes, photosensiticty etc
- Reversible arthopathy: Get’s into the joints, breaking them?
- Abnormal bone & cartilage formation: you don’t give the drug to children and pregnant women
Resistance
- Altered target: there are chromosomal mutations in the bacterial genes that lowers the affinity for fluoroquinolones.
- Decrease accumulation: due to porin channels and efflux pumps.
Inhibition of nucleotide synthesis: Sulphonamides
General facts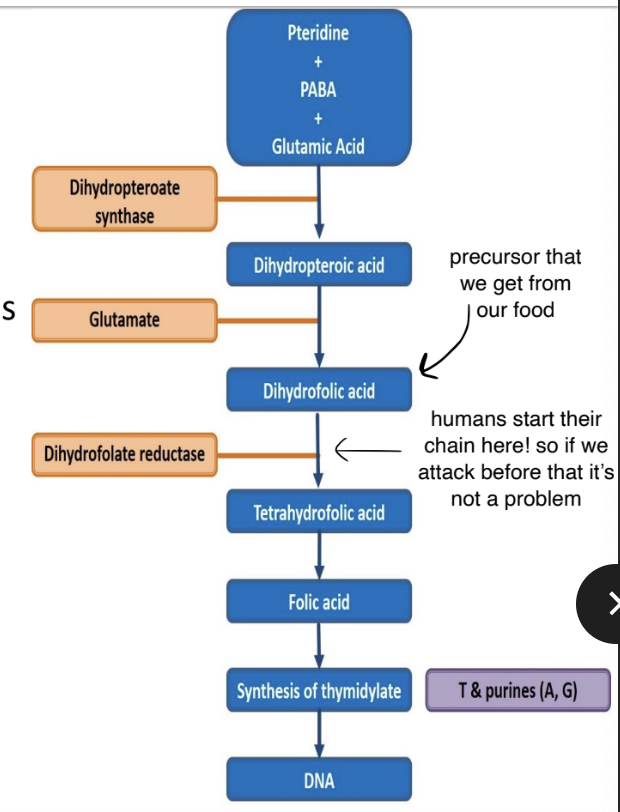
- Folic acids is needed for synthesis of precursors of DNA & RNA in both humans and bacteria.
- Bacteria make their own from PABA (p-amino-benzoix acid)
- Humans used precursors that we take up from our diet
- We are therefore unaffected by anti-folate metabolites
- Sulphonamides have given rise to several important drugs, e.g. acetazolmide
- They are divided into different groups, depending on if they are short-, intermediate-, or long-lasting.
- Bacteriostatic, but can be bactericidal at higher concentrations
Mechanism of Action
- The sulphonamide part of the molecule resembles PABA.
- It inhibits binding of PABA with dihydropteroate synthase → prevents the forming of dihydropteroic acid (DHF) → inhibits folic acid formation
Pharmacokinetics:
- Administration - Orally, IV, topically (used for bad burns) - can cause reactions
- Absorption - Most cross GIT, reach max concentrations in plasma 4-6hrs
- Distribution - up to 90% bound to albumin, widely distributed, crosses BBB, crosses placenta, reaches inflammatory sites
- Metabolism - acetylated and conjugated primarily in liver
- Excretion - unchanged are eliminated via glomerular filtration & secretion, can be excreted in breast milk
Spectrum of activity
- Baceteriostatic against many G+ and G- bacteria
- E.g. streptococcus and enterobacteria, chlamydia etc
- → stimulates growth of rickettsiae
Clinical use
- Only used in drug combination:
- Urinary tract infections: sulfasalazine
- GIT disorders: Sulfasalazine for IBS :O
Side effects
- GIT: Nausea, vomiting diarreah
- Nephrotoxicity - renal obstruction
- Hypersensiticity reactions - rashes, fever
- Haematological toxicity: anemia
- Kerniciterus: bilirubin induced brain dysfunction
- Hepatotoxicity: jaundice
Resistance
- Many are resistant to sulphonamides
- The resistant bacteria either: overproduce PABA, have low affinity for the DHS enzyme, adopt an alternative pathway in folate metabolism or loss of permeability to sulphonamides.
- When a bacteria becomes resistant to one sulphonamide: it becomes resistant to all :(
Drugs affecting cell membrane function
Disorganize membrane structure: Tyrocidines & Gramicidin
General facts
- Both contain the amino acid Ornithine which is not found in human proteins.
- Affects membrane structure
Mechanism of Action
- It acts as an ionophore on the bacterial cell wall - meaning that it facilitates ion transport over the membrane.
- It creates a sort of pore in which cl- and Na+ move through.
- This will disrupt the cell homeostasis, and therefore results in bacterial cell death.
Side effects
- Since it is not selective, it will cause toxicity in humans as well
Clinical use
- Primarly used in the treatment of infected surface wounds
Alter membrane permeability: Polyene antibiotics
- E.g. ionophores: valinomycin and nonactin
Alter membrane enzyme systems: All antifungals
- E.g. Azoles which have an effect on gram+ bacteria due to high levels of free fatty acids in the membranes
Inhibitors of cell wall synthesis
Beta lactams: Penicillin & cephalosporins
General facts
- There are four categories of beta-lactams:
- Penicillin
- Cephalosporins
- Monobactams
- Carbapenems
- The beta-lactam has a Thiazolidine ring which is its weakness - the bacteria will break this bond which will cause resistance.
Mechanism of Action
- Interfere with synthesis of cell wall component peptidoglycan
- It inhibits the transpeptidation enzyme which cross-links peptide chains attached to the backbone of the peptidoglycan
- Leads to a weaker cell, and therefore cell death
- Is bactericidal
Pharmacokinetics
- AD: Oral, I.V. (for some types), I.M.
- AB: Not great in GIT, food decreases absorption - should be taken on an empty stomach.
- DI: wide, no BBB (except meninges are inflamed),
- ME: metabolism can occur with impaired renal function, oxacillin metabolized in liver.
- EX: Plasma T1/2 < 2 hours, kidney
Spectrum of activity
Is dependent on which type of penicillin:
- Natural penicillin: Against non-beta-lactamase producing G+, e.g. streptococci
- Anti-staphylococcal penicillin: Active against beta-lactamase producing staphylococci
- Aminopenicillins: More active against enterococci and listeria monocytogenes
- Extended-spectrum penicillin: Great activity against G- bacteria, escp. pseudomonas species.
Clinical uses
- Are enormous, but there’s a lot of resistance.
- Examples are: pneumonia, streptococcal, meningitis, UTI’s etc
Side effects
- Hypersensiticty reactions (~10%!)
- GIT disturbances
- Nephritis
- Neurotoxicity: can provoke seizures
- Hematologic toxicity: decreased coagulation
- Some drug intereactions e.g. contraceptive pill
Cephalosporins
General facts
- Related structurally & functionally to penicillins
- Bactericidal
Spectrum of activity
- More active against gram-negative
- They have no activity against LAME - listeria, atypicals (mycoplasma&chlamydia), MRSA and Enterococci.
Pharmacokinetics
- Route of administration: Injection
- Distribution: well distributed, can cross BBB
- Elimination: kidneys
Clinical uses
- pneumonia, sepsis, UTI, meningitis etc
- Treatment of unknown bacteria
- For bacteria which are resistance to beta-lactams
Side effects
- Allergic reactions
- Cross reactivity with penicillin
- GIT disturbances
- Hematological effects (lower prothrombin)
Anti-mycobacterial agents
Inhibition of synthesis of mycollic acids: Isoniazid
Inhibition of RNA synthesis: Rifampicin
Antibiotic resistance
Definition
Antibiotic resistance has been stated to be one for the greatest threats to human health by the WHO. It is estimated to be 10 million deaths due to antimicrobial resistance by 2050 if new types of antibiotics aren’t found.
Drug resistance is the development of an organism's defenses against chemotherapeutic agents, which then will restrict the medical treatment of many infections.
Arise
There are many reasons as to why antibiotic resistance arises. The biggest factor is due to misuse and overuse of antibiotics. These include:
- Giving antibiotics to patients for the wrong disease, e.g. virus infections
- Giving broad-spectrum antibiotics instead of narrow spectrum
- Not completing the course of a treatment of the patient: some bacteria might survive and therefore become resistant
- Use of antibiotics in agriculture as a preventative measurement. This can cause the livestock to have resistant bacteria, which then can transfer to humans due to us eating the meat.
Spread
The spread of antibiotic resistances can happen in:
Person to person: someone with resistant bacteria gives a cold to another person.
Bacteria to bacteria: will spare a plasmid which contains resistant genes.
Within the bacteria: Jumping genes, which will go between chromosomes and plasmids via transposons. This is how the plasmid will be incorporated into the bacterial genome, or spread further. Transposons are easily transferable DNA segments.
Transfer between bacteria
Bacteria can transfer resistant genes between themselves. There are several ways they can do this: conjugation, transduction and transformation.
Conjugation
is the main mechanism of resistant gene transfer. It is done by cell to cell contact between two bacteria using pili. It can happen across species barriers.
The bacteria connect their pilus to create a bridge in between themselves. Then a conjugative plasmid with resistant genes are transferred through transcription using a relaxosome and a transfersome. When the bacteria then break contact they both have a copy of the resistant plasmid.
Transduction
Transduction is when a plasmid is enclosed in a bacteriophage and then is transferred to another bacteria of the same species. It’s quite ineffective, but is used a lot in staphylococci and streptococci.
Transformation
Is a gene transfer which is commonly used in research, but not as commonly between bacteria to share resistant genes. The bacteria basically take up “naked DNA” from the environment, which was released by another cell (usually through cell lysis). The DNA then gets incorporated into the genome by a cross-over mechanism. It only happens if the DNA is similar or identical to the bacteria that takes it up.
Which are the biochemical mechanisms? (four)
There are several biochemical mechanisms of resistances which have developed, and they are adapted depending on the drug. The four main mechanisms are:
- Enzymatic inactivation of the drug
- Altered drug binding site
- Decreased drug accumulation
- Development of alternative resistance pathways
Enzymatic inactivation of the drug
The bacteria have evolved several ways to enzymatically inactive the drugs.
- Beta-lactamases are enzymes which inactivate penicillins and cephalosporins by cleaving the beta-lactam ring. These are encoded on plasmids which are transferred by transduction in staphylococcus gram negative bacteria. A solution to this problem would be to co-administer the antibiotic with inhibitors of beta-lactamase
- Acetyltransferases are enzymes which inactivate chloramphenicol (CAT) (50S). These are encoded by plasmids.
- Phosphorylation / adenylation / acetylation are ways that aminoglycosides can be inactivated. The resistance genes are carried on plasmids and on transposons. These are found in both gram+ and gram- bacteria.
Altered drug binding site
Certain bacteria will mutate their binding site so the drug has a lower affinity to bind.
- The 30S binding site for aminoglycosides has been altered in some bacteria, as encoded on chromosome
- the 50S binding site for macrolides has been mutated on plasmids
- The DNA gyrase A have a point mutation, which disrupts binding for quinolones
- RNA polymerase has been altered, disrupting rifampicin
- The B-lactam binding site, where penicillin normally would bind, has been mutated on chromosome.
Decreased drug accumulation
These are mechanisms that can cause resistance against several drugs.
- Increased efflux mechanisms, which will pump out the drugs from the bacteria. These have caused resistance against tetracyclines, erythromycins and fluoroquinolones.
- Altered permeability of the cell wall, due to chromosomal mutations in polysaccharides will cause resistance to ampicillin.
Development of alternative resistance pathways
Examples of these are:
- Change of the enzyme dihydrofolate reductase so that the trimethoprim drug cannot recognize it anymore.
Summary