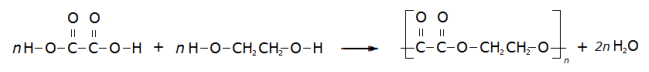Chemistry IGCSE
Spelling does not matter as long as meaning is not changed
If done know what observation says bubbles
What should the person do to make the experiment better: Repeat
2 Hours
1 hour 15 min
Ammonia in NH3 and Ammonium is NH4+
Acids: proton donors because they have H+ ions
Bases: proton acceptors as they ionize
Preparation of soluble salt from insoluble base:
Mix the insoluble base and the acid
Add excess solid (to ensure all the acid has reacted)
Filter to remove the excess solid (you are not left with a solution of your product dissolved in water)
Evaporate most of the water until crystalls begin to appear
Leave to allow to crystallize slowly
Dry the crystals in warm oven (dry oven to dry faster)
It is important to know that excess solid is added to make sure ALL acid has reacted
Preparation of a soluble salt from an acid and alkali:
Use a pipette to transfer the acid to a conical flask
Add a few drops of indicator
Place the alkali solution in the burette
Add the alkali from the burette into the acid, until the solution is neutral. Record the volume.
Repeat but without indicator using volume from step 4 (indicator will be impurity)
Evaporate most of the water until crystals begin to appear
Leave to allow to crystallise slowly
Dry the crystals in a warm oven
Step 1-4: to find the exact volume needed to netralise which is shown through the indicator
Preparation of an insoluble salt from two soluble (solution):
Mix the two solutions, a precipitate will form (the precipitate is the insoluble product)
Filter the mixture to separate the solid
Wash the solid with distilled water to remove impurities
Leave the solid on filter paper to dry
Dry the crystals in a warm oven
Allow to dry to constant mass
Method:
Matching its name a hydrocarbon is a compound that is made of of however many hydrogen and carbon ONLY
Empirical Formula: The simplest possible ratio of the atoms/elements in the molecule
e.g. Ethane is C2H6 but its empirical formula is CH3
Molecular Formula: The actual “formula“ of the molecule, shows the actual number of atoms
e.g. Butene is C4H8
General Formula: The ratio of the atoms in a family of compounds in terms of “n“
Alkandes general formula is CnH2n+2 while Alkenes general formula is CnH2n
Structural Formula: Displays enough information to make the structure clear but does not show the bonds

Displayed Formula: shows the spatial arrangement of all atoms, molecule, and bonds

Homologous Series: A collection/series/family of organic compounds that have the same chemical properties due to having the same functional group
All compounds within the same homologous series have:
The same general formula
The same functional group
Similar chemical properties
Physical properties that change gradient
The difference between each consecutive member is CH2
Functional Group: A group of atoms bonded together in a very specific arrangement that influences what homologous series they are a a part of (the part of the compoud that makes it that specific homologous series/ the characteristic that makes you part of the homologous series)

Isomer: Compounds that have the same molecular formula but different displayed formula
Naming an organic compound has two parts
The number of carbon atoms present in the compound
Name | Number of Carbons |
|---|---|
Meth… | 1 |
Eth… | 2 |
Prop… | 3 |
But… | 4 |
Pent… | 5 |
Hex… | 6 |
The homologous series it is part of
Name | Homologous Series | Functional Group |
|---|---|---|
…ane | Alkane | - |
…ene | Alkene | C = C Bond |
…anol | Alcohol | - OH |
…anoic acid | Carboxylic Acid | - C = O - OH |
…amine | Amine | -NH2 |
…yl …anoate | Ester | - C = O - O - |
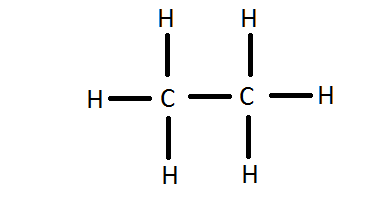
If the molecular formula of a compound is C2H6, structural formula is CH3CH3
Substitution: a reaction that takes place when one functional group is replaced by another
Methane reacts with bromine under ultraviolet light
CH4 + Br2 → CH3Br + HB
Methane + Bromine → Bromomethane + Hydrogen Bromide
Addition: a reaction that takes place when two or more molecules combine to form a larger molecule with no other product
Bromine will react with ethene and the bromine molecule will react and add across the double bond of the ethene
C2H4 + Br2 → C2H4Br2
Ethene + Bromine → Dibromoethane
Combustion: a reaction where an organic substance reacts with oxygen to form carbon dioxide and water
Complete Combustion: If there is an unlimited supply of oxygen, the products are carbon dioxide and water:
CH4 + 2O2 → CO2 + 2H2O
C3H8 + 5O2 → 3CO2 + 4H2O
Incomplete combustion: If there is a limited supply of oxygen, the products are carbon monoxide and water:
CH4 + O2 → CO + 2H2O
Crude Oil: a mixture of different hydrocarbons called fractions (together the substance is not very useful)
A finite resource
The fractions in crude oil are separated through the process of fractional distillation.
The size and length of each hydrocarbon molecule determine which fraction it is separated into
Inside the fractional column, the crude oil is heated
The oil evaporates up the column
The oil condenses as it goes up the column
The hydrocarbons with the highest boiling point condense first, at the bottom of the column (bigger)
Hydrocarbons with the lowest boiling point condense last, at the top of the column (small)
From Top to Bottom (low BP to high BP)
Refinery Gases
Domestic Heating
Cooking
Gasoline/Petrol:
Fuel for cars
Kerosene:
Aircratf fuel
Diesel:
Fuel for cars and buses
Fuel Oil:
Fuel for large ships and power stations
Bitumen (Bottom): Bitume for roads and roofs

Viscosity: how easily can the liquid flow. The number of hydrocarbons increases → attraction between hydrocarbons increases → liquid becomes more vicious
Colour: As carbon chain length increases → colour get darker (thicker)
MP and BP: As molecules get larger → intermolecular attraction becomes greater → more heat is needed to seperate the molecules → increased BP and MP
Volatility: tendency of a substance to vaporise. Increased molecular size → hydrocarbon liquid becomes less volatile (because the intermolecular attraction increases with increased size of molecules)
Sum: As you go up the column BP and viscosity decreases
Fuel: substance that releases heat energy when burned
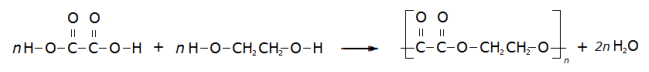
Spelling does not matter as long as meaning is not changed
If done know what observation says bubbles
What should the person do to make the experiment better: Repeat
2 Hours
1 hour 15 min
Ammonia in NH3 and Ammonium is NH4+
Acids: proton donors because they have H+ ions
Bases: proton acceptors as they ionize
Preparation of soluble salt from insoluble base:
Mix the insoluble base and the acid
Add excess solid (to ensure all the acid has reacted)
Filter to remove the excess solid (you are not left with a solution of your product dissolved in water)
Evaporate most of the water until crystalls begin to appear
Leave to allow to crystallize slowly
Dry the crystals in warm oven (dry oven to dry faster)
It is important to know that excess solid is added to make sure ALL acid has reacted
Preparation of a soluble salt from an acid and alkali:
Use a pipette to transfer the acid to a conical flask
Add a few drops of indicator
Place the alkali solution in the burette
Add the alkali from the burette into the acid, until the solution is neutral. Record the volume.
Repeat but without indicator using volume from step 4 (indicator will be impurity)
Evaporate most of the water until crystals begin to appear
Leave to allow to crystallise slowly
Dry the crystals in a warm oven
Step 1-4: to find the exact volume needed to netralise which is shown through the indicator
Preparation of an insoluble salt from two soluble (solution):
Mix the two solutions, a precipitate will form (the precipitate is the insoluble product)
Filter the mixture to separate the solid
Wash the solid with distilled water to remove impurities
Leave the solid on filter paper to dry
Dry the crystals in a warm oven
Allow to dry to constant mass
Method:
Matching its name a hydrocarbon is a compound that is made of of however many hydrogen and carbon ONLY
Empirical Formula: The simplest possible ratio of the atoms/elements in the molecule
e.g. Ethane is C2H6 but its empirical formula is CH3
Molecular Formula: The actual “formula“ of the molecule, shows the actual number of atoms
e.g. Butene is C4H8
General Formula: The ratio of the atoms in a family of compounds in terms of “n“
Alkandes general formula is CnH2n+2 while Alkenes general formula is CnH2n
Structural Formula: Displays enough information to make the structure clear but does not show the bonds

Displayed Formula: shows the spatial arrangement of all atoms, molecule, and bonds

Homologous Series: A collection/series/family of organic compounds that have the same chemical properties due to having the same functional group
All compounds within the same homologous series have:
The same general formula
The same functional group
Similar chemical properties
Physical properties that change gradient
The difference between each consecutive member is CH2
Functional Group: A group of atoms bonded together in a very specific arrangement that influences what homologous series they are a a part of (the part of the compoud that makes it that specific homologous series/ the characteristic that makes you part of the homologous series)

Isomer: Compounds that have the same molecular formula but different displayed formula
Naming an organic compound has two parts
The number of carbon atoms present in the compound
Name | Number of Carbons |
|---|---|
Meth… | 1 |
Eth… | 2 |
Prop… | 3 |
But… | 4 |
Pent… | 5 |
Hex… | 6 |
The homologous series it is part of
Name | Homologous Series | Functional Group |
|---|---|---|
…ane | Alkane | - |
…ene | Alkene | C = C Bond |
…anol | Alcohol | - OH |
…anoic acid | Carboxylic Acid | - C = O - OH |
…amine | Amine | -NH2 |
…yl …anoate | Ester | - C = O - O - |
If the molecular formula of a compound is C2H6, structural formula is CH3CH3

Substitution: a reaction that takes place when one functional group is replaced by another
Methane reacts with bromine under ultraviolet light
CH4 + Br2 → CH3Br + HB
Methane + Bromine → Bromomethane + Hydrogen Bromide
Addition: a reaction that takes place when two or more molecules combine to form a larger molecule with no other product
Bromine will react with ethene and the bromine molecule will react and add across the double bond of the ethene
C2H4 + Br2 → C2H4Br2
Ethene + Bromine → Dibromoethane
Combustion: a reaction where an organic substance reacts with oxygen to form carbon dioxide and water
Complete Combustion: If there is an unlimited supply of oxygen, the products are carbon dioxide and water:
CH4 + 2O2 → CO2 + 2H2O
C3H8 + 5O2 → 3CO2 + 4H2O
Incomplete combustion: If there is a limited supply of oxygen, the products are carbon monoxide and water:
CH4 + O2 → CO + 2H2O
Crude Oil: a mixture of different hydrocarbons called fractions (together the substance is not very useful)
A finite resource
The fractions in crude oil are separated through the process of fractional distillation.
The size and length of each hydrocarbon molecule determine which fraction it is separated into
Inside the fractional column, the crude oil is heated
The oil evaporates up the column
The oil condenses as it goes up the column
The hydrocarbons with the highest boiling point condense first, at the bottom of the column (bigger)
Hydrocarbons with the lowest boiling point condense last, at the top of the column (small)
From Top to Bottom (low BP to high BP)
Refinery Gases
Domestic Heating
Cooking
Gasoline/Petrol:
Fuel for cars
Kerosene:
Aircratf fuel
Diesel:
Fuel for cars and buses
Fuel Oil:
Fuel for large ships and power stations
Bitumen (Bottom): Bitume for roads and roofs

Viscosity: how easily can the liquid flow. The number of hydrocarbons increases → attraction between hydrocarbons increases → liquid becomes more vicious
Colour: As carbon chain length increases → colour get darker (thicker)
MP and BP: As molecules get larger → intermolecular attraction becomes greater → more heat is needed to seperate the molecules → increased BP and MP
Volatility: tendency of a substance to vaporise. Increased molecular size → hydrocarbon liquid becomes less volatile (because the intermolecular attraction increases with increased size of molecules)
Sum: As you go up the column BP and viscosity decreases
Fuel: substance that releases heat energy when burned