Lecture 10 ECM and Cytoskeleton
Learning Objectives
Nature and Role of ECM Proteins: Understand ECM components including collagen.
Collagen Types: Differentiate between various types of collagen.
Integrin Conformations: Identify roles that different conformations of integrins play in mammalian cells.
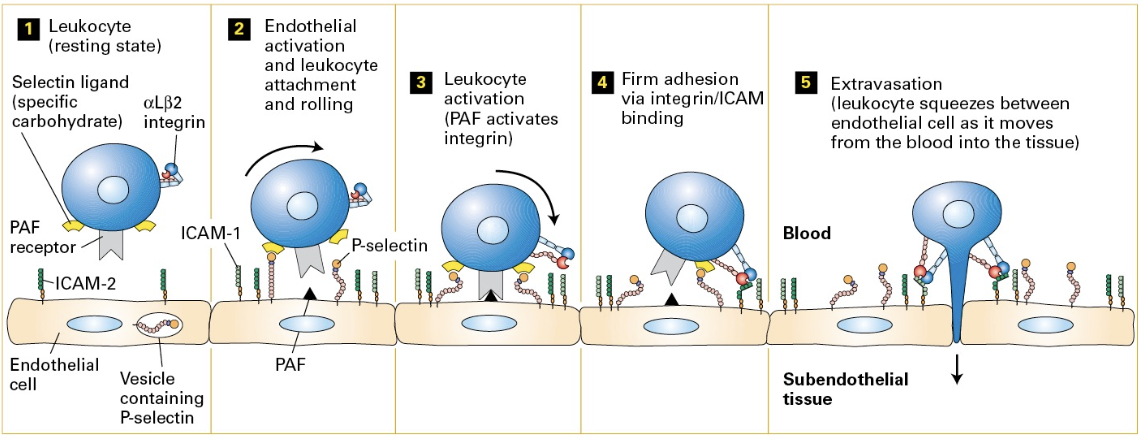
Adhesion Molecules: Focus on the steps and functions of adhesion molecules in phenomena like extravasation.
Plasmodesmata vs Intercellular Adhesion: Differentiate their roles in plant and mammalian cells.
Intermediate Filaments: Characterize and differentiate the functions and distributions of various intermediate filaments.
Selected Collagens - Table 20.5
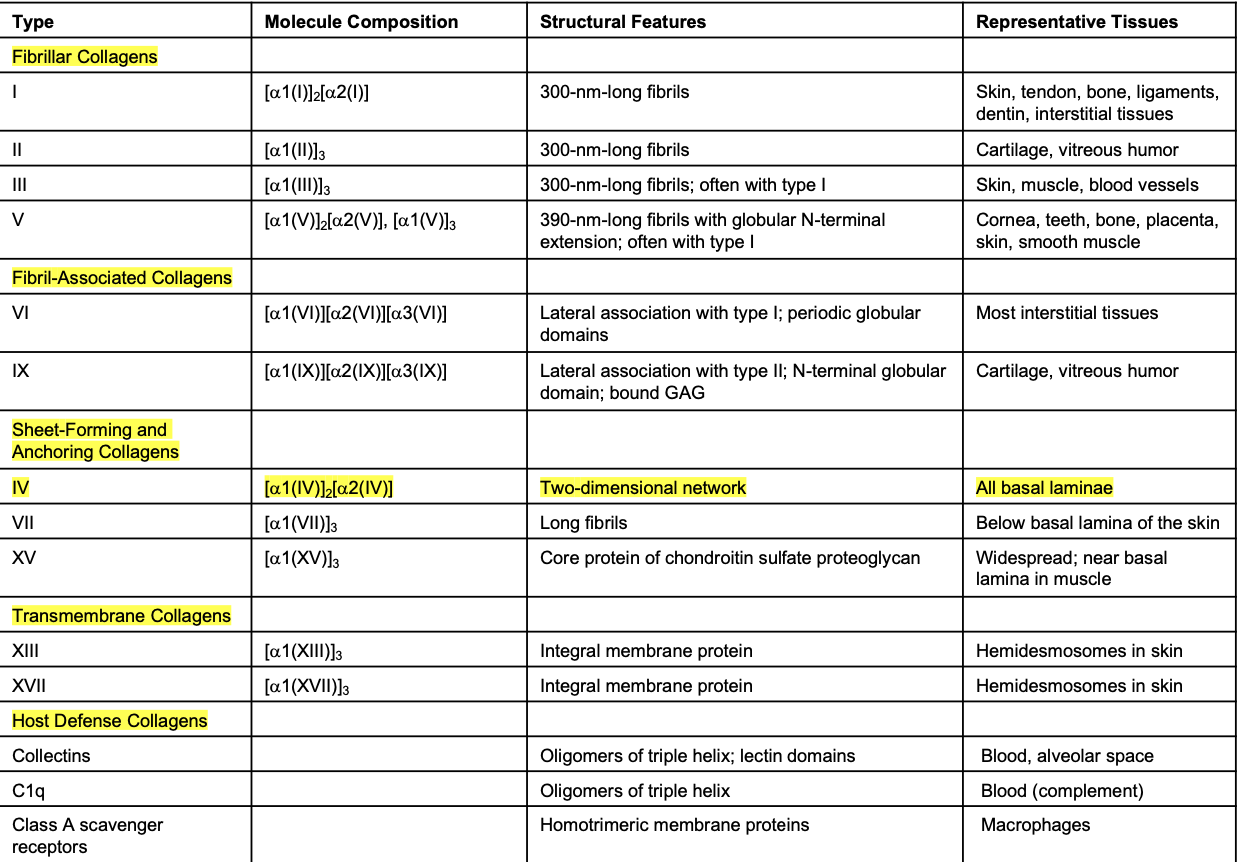
Page 4: Type IV Collagen Structure
Found in all Basal Laminae
Characteristics: 400-nm long triple helical structures with 24 non-helical interruptions adding flexibility.
Association and Network Formation: Flanked by globular domains for interaction; covalently cross-linked to form networks, attaching to laminin to create the basal lamina.
Perlecan (proteoglycan)
Description: Major secreted proteoglycan ECM component within basal laminae.
Structure: Large multidomain core protein with multiple polysaccharide side-chains.
Function: Binds with various other molecules like nidogen and laminin to mediate cell-surface receptor interactions.
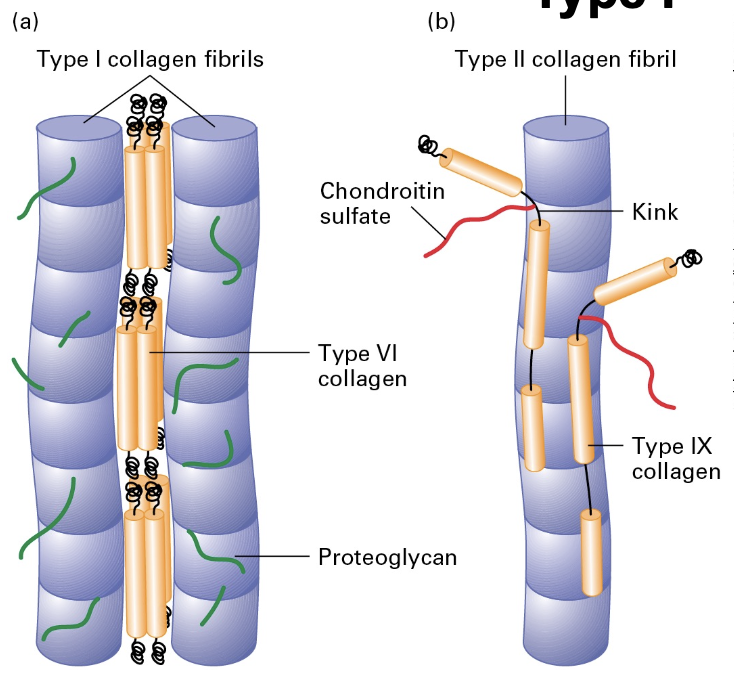
Collagen Structure and Assembly
Triple-Helical Structure: Assembles characterized by G - X - Y repeat motif where G is glycine (central), X is often proline, and Y is hydroxyproline.
Fiber Formation: Microfibrils (300 nm) aggregate to form fibrils and further bundle into long fibers.
Type I Properties
Fibrillar Molecules: Most abundant; largely present in connective tissues (Types I, II, III).
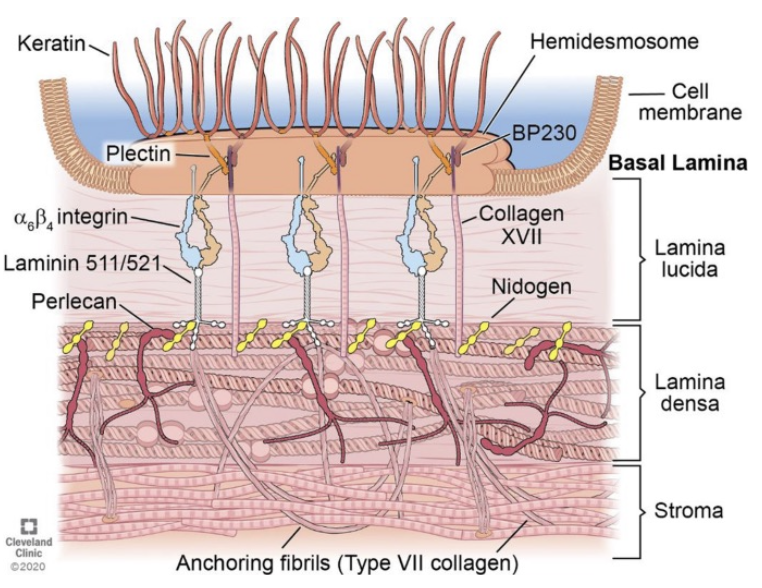
Fibril-Associated Collagens: Maintain structural integrity by bridging fibrillar collagens with each other or to the ECM.
Sheet-Forming Collagens: Type IV forms 2D networks crucial to basal laminae.
Type I Collagen: Known for exceptional tensile strength, used heavily in bone reinforcement (30% protein, 70% mineral). Stronger than steel, gram for gram
Collagen Synthesis and Assembly
Collagen Production: Occurs in the rough endoplasmic reticulum (ER), Golgi apparatus, and extracellular space.
Procollagen to Collagen Molecule: Involves propeptide cleavage and lateral association leading to the assembly of collagen fibers.
Glycosaminoglycans (GAGs)
Role in ECM: Proteoglycans crucial for adhesion, existing in secreted or membrane-attached forms.
Classifications: Four types differentiated by disaccharide repeats and modifications, contributing to the structure of proteoglycans. Covalently linked to specialized polysaccharide side-chains (GAGs)
GAG Synthesis
Core Syntheses: Proteoglycan cores synthesized in the ER; GAG chains assembled in the Golgi.
Linkage Variations: GAGs may be O-linked (attached by the -OH moiety in Serine or Threonine R-groups) or N-linked (attached by the R-group of the asparagine residues).
Hyaluronan (Hyaluronic Acid, HA)
Definition: A non-sulfated GAG, significant for ECM structure, made by membrane bound HA-synthase and secreted into the ECM. Forms the backbone of ECM proteoglycan aggregates.
Characteristics: Forms hydrated spheres/gel; resists compressive forces, predominantly found in cartilage.
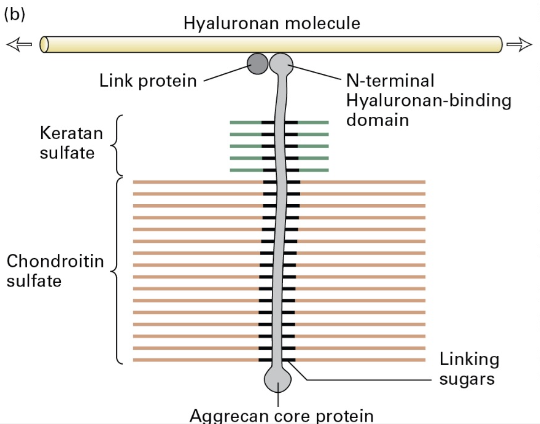
Fibronectin
Description: An important ECM component across vertebrates; composed of dimers of 2 similar polypeptides linked at C-terminus linked by disulfide bonds.
Function: Attaches cells to ECM via binding to fibrillar collagen, proteoglycans, and adhesion receptors; contains specific binding domain (RGD) for integrin.
Domains are Type I, II, or III. Arg-Gly-Asp domain binds Integrin
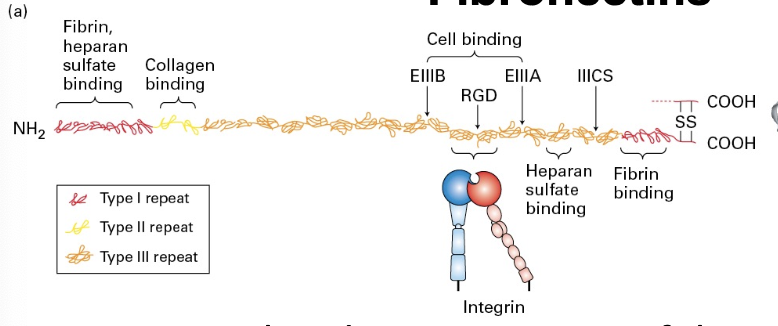
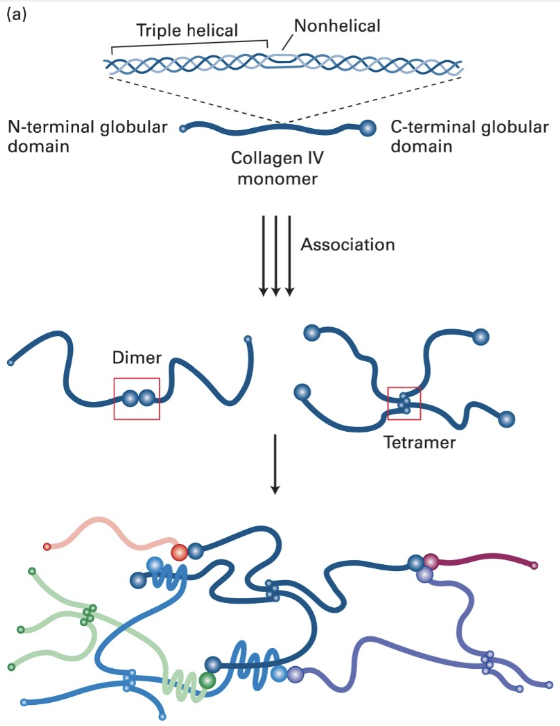
Page 13: Elastic Fibers
Structure: Composed of fibers with elastin cores; essential for tissues under mechanical strain or deformation like lungs.
Components: Elastin is covalently cross-linked tropoelastin aggregates surrounded by collagen microfibrils. Elastin core of fibers.
Matrix Metalloproteases (MMPs)
ECM Dynamics: The ECM is subject to remodeling and degradation primarily by zinc-dependent MMPs.
Subgroups of MMPs: Include MMPs for remodeling and degradation, ADAMs for integrin degradation, and ADAMTSs for signaling functions.
ADAM: Asidintegrin and metalloproteinases
Page 15: Integrins and Adhesion
Dynamic Adhesion: Integrins connect the ECM and cytoskeleton, clustering into various adhesive structures.
focal adhesions in 2D culture
3D adhesion in 3D cultures
Fibrillar adhesions
Podosomes (“foot bodies”)
Page 16: Integrin Binding Affinity States
3 Conformational States that correspond to binding affinity:
Bent Closed
Extended Closed
Extended Open
Functionality: These states correlate with binding affinity of integrins.
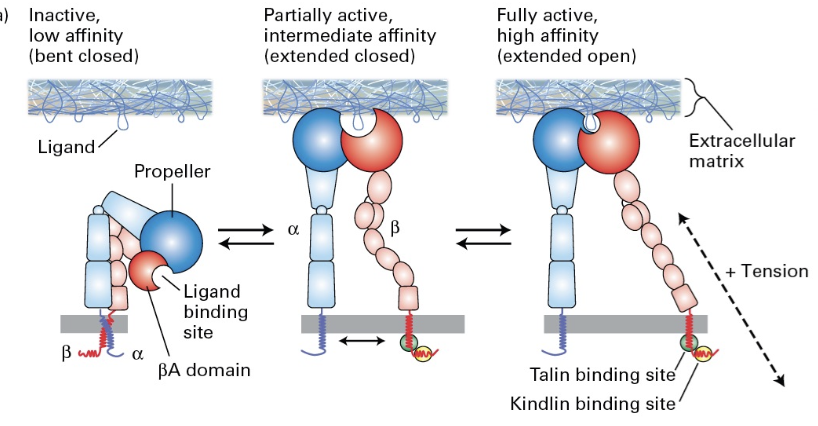
Signaling through Integrins
Cytosolic Domains: Integral proteins can signal and connect to the cytoskeleton; molecules like talin modify phospholipids and allows association, leading to integrin activation and complex formation. Association in turn causes: activation, dimerization, complex building, integrin activation, recruitment and force
Fibroblasts
Role: Major secretors of ECM; critical for maintaining ECM dynamics.
Location: Present in corneal structures, including Bowman's layer, Descemet's membrane, and stroma.
Cell Motility in Immunity
Importance: Motility vital for wound healing and immune responses; immune cells are recruited via extravasation to areas needing intervention.
Plant Cell Walls
Structure: Comprised of polysaccharides forming ECM-like structures, mainly cellulose, hemicellulose, and pectin. Cellulose is synthesized outside the cell membrane, while hemicellulose and pectin are ER synthesied
Function: Resist turgor pressure (like air filling a balloon), produced externally to the cell membrane.
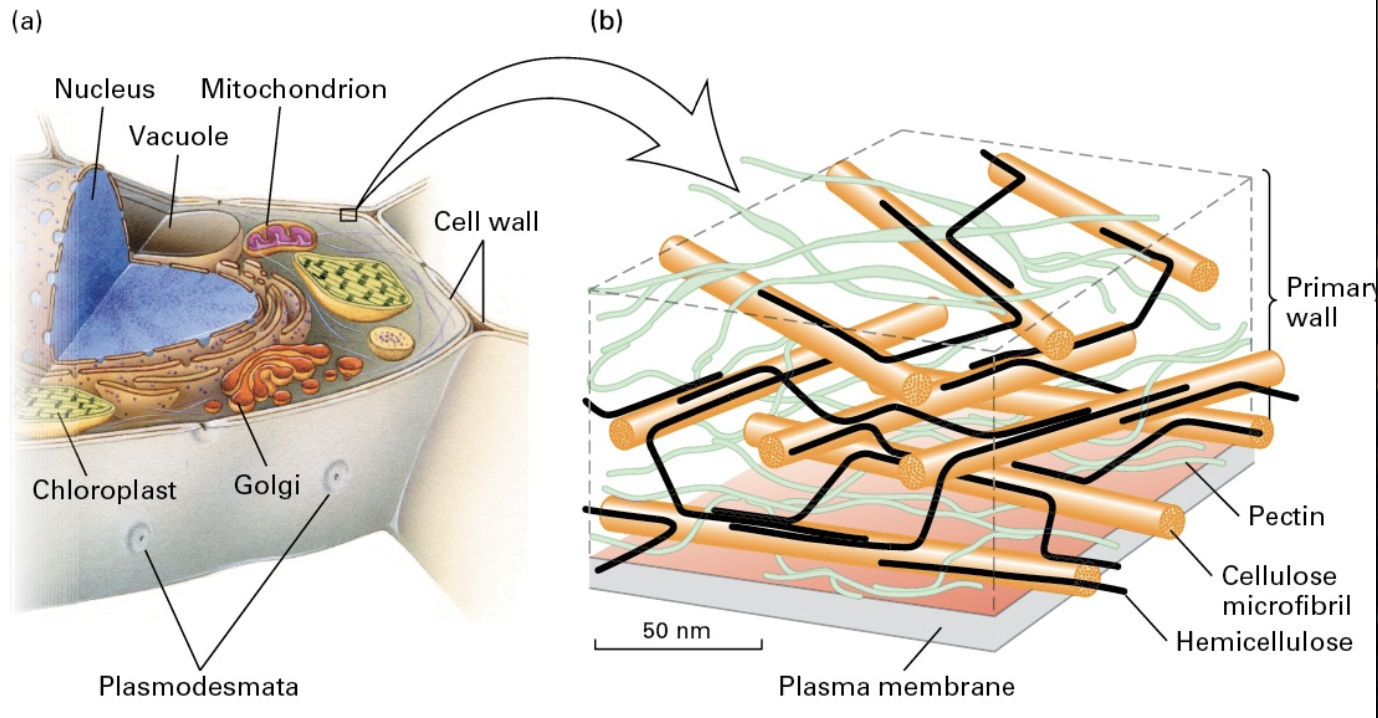
Page 21: Plasmodesmata in Plants
Description: Connect adjacent plant cells through structures that share an ER membrane; allow the transfer of molecules between cells.
Cell walls means almost no CAMs
Plants have plasmodesmata structures
that connect adjacent cells
Plasmodesmata share an ER membrane that lines the structure (the annulus) that is 30-60nm to >1μm in diameter
A narrow tube (desmotubule) is formed with a regulated gap of 2-10 nm called the cytoplasmic sleeve
Allows free transfer of small molecules or regulated transfer of large ones
Page 22: Intermediate Filaments
Characteristics: 10 nm thick, stable structures with biochemically heterogenous group, great tensile strength, no inttrinsic polarity, no motore proteins associated, much more stable than the other 2 filaments, and are not found in all eukaryotic cells.
Major Classes of Intermediate Filaments
Class
Protein
Distribution
Proposed Function
I
Acidic keratins
Epithelial cells
Tissue strength and integrity
II
Basic keratins
Epithelial cells
Tissue strength and integrity
III
Desmin, GFAP, vimentin
Muscle, glial cells, mesenchymal cells
Sarcomere organization, integrity
IV
Neurofilaments (NFL, NFM, and NFH)
Neurons
Axon organization
V
Lamins
Nucleus
Nuclear structure and organization
Structure of Intermediate Filaments
Conserved dimerized 310 aa a helical rod domain
Basic unit = dimer, dimers form anti parallel tetrads, tetrads unite to form protofilaments, 4 protofilaments unite to form a protofibril, 4 protofibril unite to form an IF
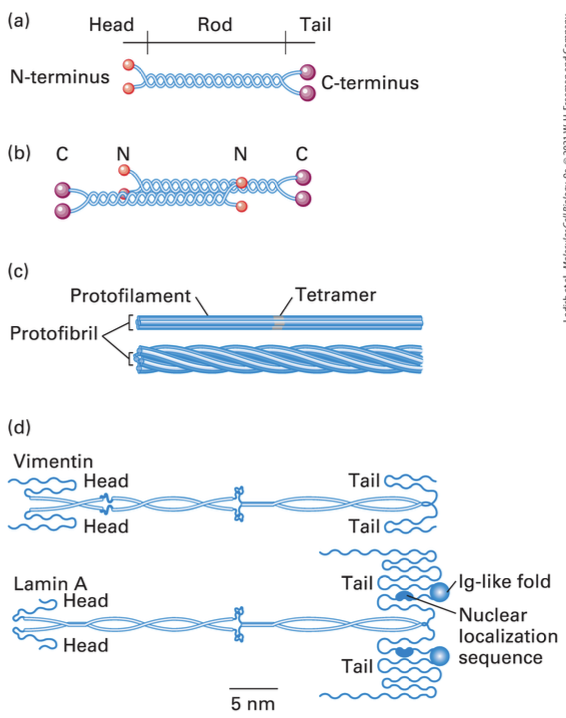
Variability in Intermediate Filaments
Structural Differences: Variances in non-helical domains complicate formation; involvement of non-nucleating, capping, sequestering, or severing proteins.
Polarity of Intermediate Filaments
Classes of Keratins make up:
Class I and II: in epithelia
Class III in mesoderm
Class IV in neurofilaments
Class V in the nucleus of all our cells
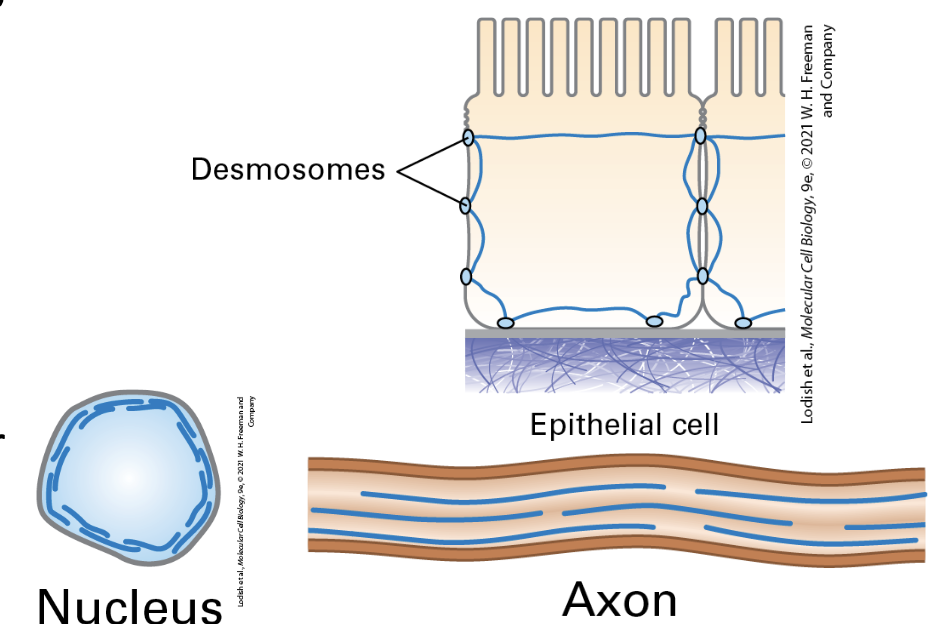
Stability of Intermediate Filaments
Dynamic Structures: Incorporation of free units into stable structures; keratin in epithelial appendages provides mechanical strength.
Free IF units can be incorporated into existing structures.
Keratin dimer units are acidic/basic pairs. Keratins make hair and nails hard. Cytokeratins strengthen connection to make a tight skin/dermal layer.
Lamins in the Nucleus
Function: Support the nuclear envelope and maintain chromatin structure; specific classes (A/C and B1/B2) with distinct associations and functions.
Lamins are the meshwork on the inside of our nuclei, connecting the membrane and chromatin
Lamin A provides rigidty
Lamin B associated with receptors and helps attach the nucleus to the cytoskeleton through SUN and KASH domain containing proteins
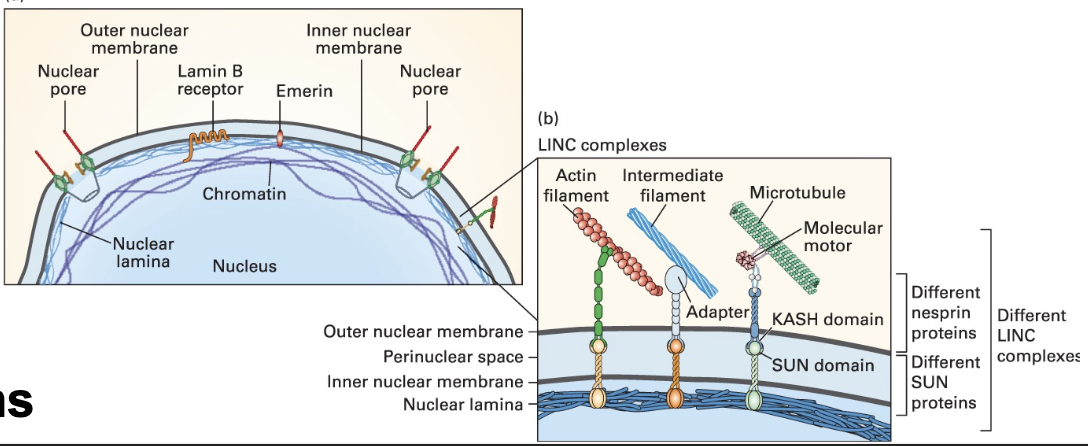
Intermediate Filament-Associated Proteins (IFAPs)
Role: Support the connections between intermediate filaments and other cytoskeletal components, enhancing cellular maintenance and integrity.
Co purify with IFs
IFAPs include plakin family members. Plakins link IF to the desmosomes and hemidesmosomes. Other plakins connect IFs to microfilaments and microtubules.