Chapter 2: ATOMS, MOLECULES, AND IONS
2.1 ∣ The Atomic Theory of Matter
Democritus (460–370 bce) and other early Greek philosophers described the material world as made up of tiny indivisible particles that they called atomos, meaning “indivisible” or “uncuttable.” Later, however, Plato and Aristotle formulated the notion that there can be no ultimately indivisible particles, and the “atomic” view of matter faded for many centuries during which Aristotelean philosophy dominated Western culture.
Dalton’s Atomic Theory
John Dalton (1766–1844), the son of a poor English weaver, began teaching at age 12. He spent most of his years in Manchester, where he taught both grammar school and college. His lifelong interest in meteorology led him to study gases, then chemistry, and eventually atomic theory. Despite his humble beginnings, Dalton gained a strong scientific reputation during his lifetime.
- Each element is composed of extremely small particles called atoms.
- All atoms of a given element are identical, but the atoms of one element are different from the atoms of all other elements.
- Atoms of one element cannot be changed into atoms of a different element by chemical reactions; atoms are neither created nor destroyed in chemical reactions.
- Compounds are formed when atoms of more than one element combine; a given compound always has the same relative number and kind of atoms.
Law of conservation of Mass
The total mass of materials present after a chemical reaction is the same as the total mass present before the reaction.
Law of multiple Proportions
If two elements A and B combine to form more than one compound, the masses of B that can combine with a given mass of A are in the ratio of small whole numbers.
2.2 ∣ The Discovery of Atomic Structure
Laboratory chemistry observations informed Dalton's atom conclusions. He explained constant composition and multiple proportions by assuming atoms. Dalton and his successors have no direct proof for atoms.
Cathode Rays and electrons
Scientists studied electrical discharge through a nearly airless glass tube in the mid-1800s. The tube electrodes radiated when high voltage was applied. Cathode rays passed from the negative electrode to the positive. Because they glow, the beams were detected even though they were invisible.
The British scientist J. J. Thomson (1856–1940) found that cathode rays are the same regardless of the cathode material. Thomson built a cathode-ray tube with an anode hole for cathode rays. The beam was perpendicular to electrically charged plates, a magnet, and a fluorescent screen that lit up when hit by a cathode ray. Since the electron is negatively charged, the electric field deflected the rays one way and the magnetic field the other. Thomson balanced the areas so electrons could travel straight to the screen.
In 1909, Robert Millikan (1868–1953) of the University of Chicago succeeded in measuring the charge of an electron by performing the experiment. He then calculated the mass of the electron by using his experimental value for the charge, 1.602 * 10-19 C, and Thomson’s charge- to-mass ratio, 1.76 * 108 C/g.
Radioactivity
In 1896 the French scientist Henri Becquerel (1852–1908) discovered that a compound of uranium spontaneously emits high-energy radiation. This spontaneous emission of radiation is called radioactivity.
Marie Sklodowska Curie (1867–1934). In 1903, Henri Becquerel, Marie Curie, and her husband, Pierre, were jointly awarded the Nobel Prize in Physics for their pioneering work on radioactivity (a term she introduced). In 1911, Marie Curie won a second Nobel Prize, this time in chemistry for her discovery of the elements polonium and radium.
Three types of Radiation (Ernest Rutherford):
**alpha (**a), beta (b), and gamma (y)
The Nuclear Model of the Atom
Scientists studied particle interactions as evidence grew that the atom is made of smaller particles. Thomson reasoned in the early 1900s that since electrons contribute just a small proportion of an atom's mass, they probably contribute an equally small fraction of its size. He argued that the atom is a uniform positive sphere of matter with uniformly distributed mass and electrons embedded like raisins in pudding or watermelon seeds. The plum-pudding model was short-lived.
In 1910, Rutherford examined the angles particles deflected or dispersed through a thin gold foil. Thomson's plum-pudding model showed that most particles passed through the foil undeflected, but a few deflected 1°. Rutherford suggested laboratory undergraduate Ernest Marsden (1889–1970) examine scattering at broad angles for completeness. Some particles returned at tremendous angles, shocking everyone. They opposed Thomson's plum-pudding hypothesis but were vague.
Rutherford explained the results by proposing the nuclear model of the atom, in which most of each gold atom's mass and positive charge are concentrated in a tiny, dense nucleus.
Experiments found protons and neutrons in the nucleus. James Chadwick discovered neutrons in 1932 and Rutherford identified protons in 1919. The atom has electrons, protons, and neutrons.
2.3 ∣ The Modern View of Atomic Structure
Electronic charge is 1.602*10^-19 C. Instead of coulombs, atomic and subatomic particle charges are frequently multiples of this charge for ease. Electrons have 1 charge and protons 1+. Neutrons are electrically neutral. Atoms have no net electrical charge since they have equal electrons and protons.
Most atoms have diameters between 1 * 10^-10 m 1100 pm2 and 5 * 10^-10 m 1500 pm2. A convenient non-SI unit of length used for atomic dimensions is the angstrom 1A° 2, where 1 A ° = 1 * 10^-10 m = 100 pm. Thus, atoms have diameters of approximately 1 - 5 A.
- Electrons are attracted to the protons in the nucleus by the electrostatic force that exists between particles of opposite electrical charge.
- Atoms have extremely small masses.
- We use the atomic mass unit (amu).
| Comparison of the Proton, Neutron, and Electron | ||
|---|---|---|
| Particle | Charge | Mass (amu) |
| Proton | Positive (1+) | 1.0073 |
| Neutron | None (neutral) | 1.0087 |
| Electron | Negative (1-) | 5.486 * 10^-4 |
Atomic Numbers, Mass Numbers, and Isotopes
The atoms of each element have a characteristic number of protons. The number of protons in an atom of any particular element is called that element’s atomic number. Because an atom has no net electrical charge, the number of electrons it contains must equal the number of protons.
Atoms of a given element can differ in the number of neutrons they contain and, consequently, in mass. The atomic number is indicated by the subscript; the superscript, called the mass number, is the number of protons plus neutrons in the atom. Because all atoms of a given element have the same atomic number, the subscript is redundant and is often omitted. Atoms with identical atomic numbers but different mass numbers (that is, the same number of protons but different numbers of neutrons) are called isotopes of one another.
2.4 ∣ Atomic Weights
- Scientists of the nineteenth century were aware that atoms of different elements have different masses.
- Once scientists understood that water contains two hydrogen atoms for each oxygen atom, they concluded that an oxygen atom must have 2 * 8 = 16 times as much mass as a hydrogen atom.
- Hydrogen, the lightest atom, was arbitrarily assigned a relative mass of 1 (no units).
- It is convenient to use the atomic mass unit when dealing with these extremely small masses.
The Atomic Mass Scale
Atomic Weight
Most elements occur in nature as mixtures of isotopes. We can determine the average atomic mass of an element, usually called the element’s atomic weight, by summing (indicated by the Greek sigma, g) over the masses of its isotopes multiplied by their relative abundances.
- The most accurate means for determining atomic weights is provided by the mass spectrometer.
- A graph of the intensity of the detector signal versus ion atomic mass is called a mass spectrum.
- The gas-phase species must be converted to positively charged particles called ions.
2.5 ∣ The Periodic Table
Many elements show strong similarities to one another. The elements lithium (Li), sodium (Na), and potassium (K) are all soft, very reactive metals, for example. The ele- ments helium (He), neon (Ne), and argon (Ar) are all nonreactive gases. If the elements are arranged in order of increasing atomic number, their chemical and physical properties show a repeating, or periodic, pattern.
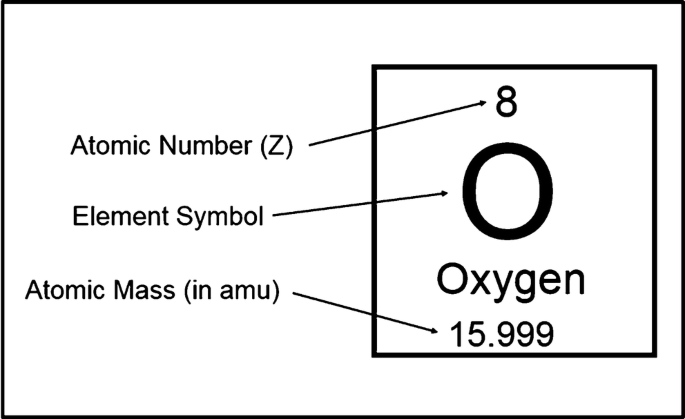
Periodic Table
The arrangement of elements in order of increasing atomic number, with elements having similar properties placed in vertical columns.
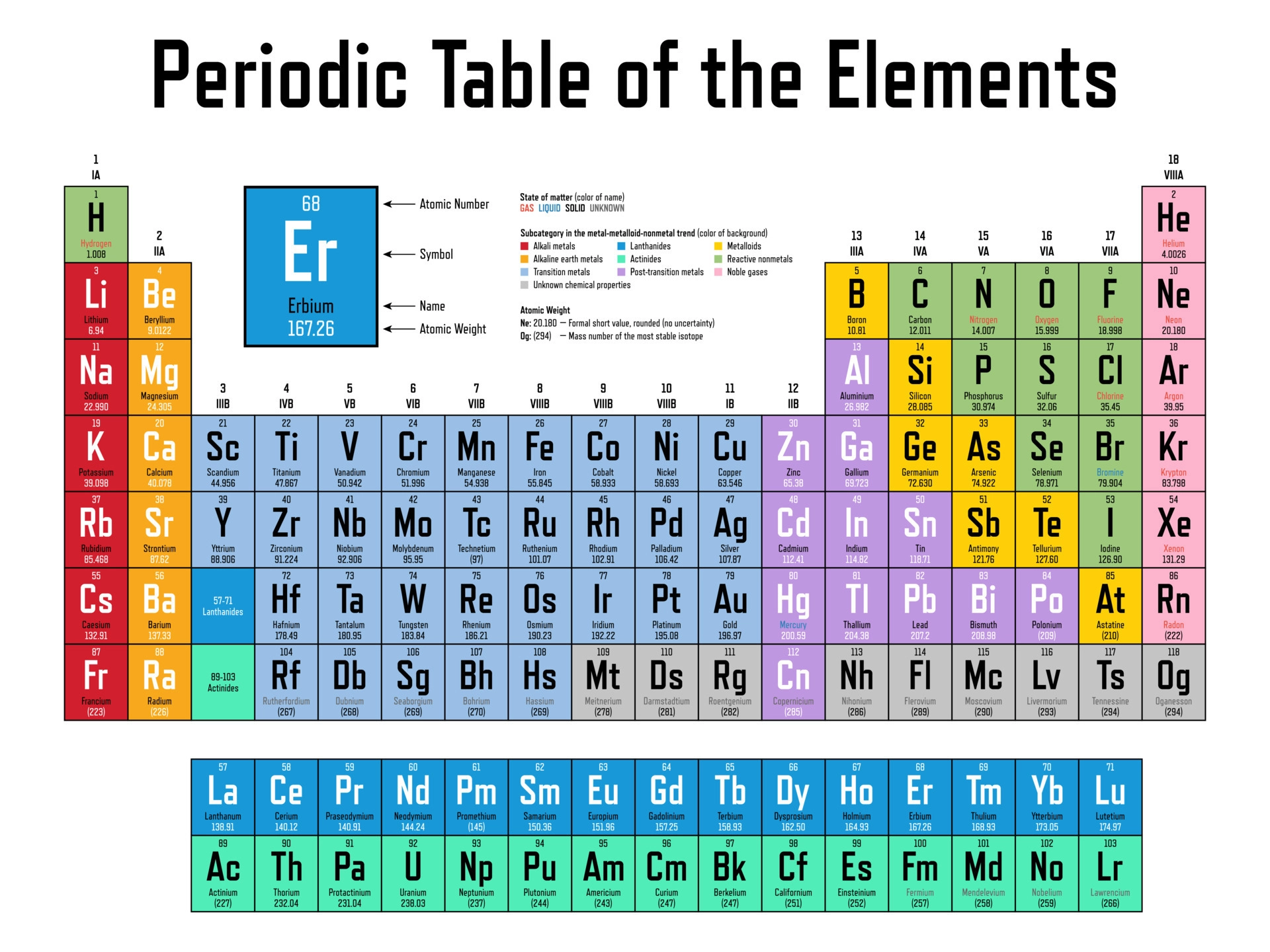
The horizontal rows of the periodic table are called periods. The first period consists of only two elements, hydrogen (H) and helium (He). The second and third periods consist of eight elements each. The fourth and fifth periods contain 18 elements. The sixth and seventh periods have 32 elements each, but in order to fit on a page 14 of the elements from each period (atomic numbers 57–70 and 89–102) appear at the bottom of the table.
The vertical columns are groups. The way in which the groups are labeled is somewhat arbitrary.
Elements in a group often exhibit similarities in physical and chemical properties.
All the elements on the left and in the middle of the table are metallic elements, or metals.
The metals are separated from the nonmetallic elements, or nonmetals, by a stepped line that runs from boron (B) to astatine (At).
Nonmetals generally differ from metals in appearance and in other physical properties. Many of the elements that lie along the line that separates metals from nonmetals have properties that fall between those of metals and nonmetals. These elements are often referred to as metalloids.
| Names of Some Groups in the Periodic Table | ||
|---|---|---|
| Group | Name | Elements |
| 1A | Alkali metals | Li, Na, K, Rb, Cs, Fr |
| 2A | Alkaline earth metals | Be, Mg, Ca, Sr, Ba, Ra |
| 6A | Chalcogens | O, S, Se, Te, Po |
| 7A | Halogens | F, Cl, Br, I, At |
| 8A | Noble gases | He, Ne, Ar, Kr, Xe, Rn |
2.6 ∣ Molecules and Molecular Compounds
Molecules and Chemical Formulas
- Several elements are found in nature in molecular form—two or more of the same type of atom bound together.
- A molecule made up of two atoms is called a diatomic molecule.
- Oxygen also exists in another molecular form known as ozone. Molecules of ozone consist of three oxygen atoms, making the chemical formula O3.
- The elements that normally occur as diatomic molecules are hydrogen, oxygen, nitrogen, and the halogens.
- Compounds composed of molecules contain more than one type of atom and are called molecular compounds.
Molecular and empirical Formulas
- Chemical formulas that indicate the actual numbers of atoms in a molecule are called molecular formulas.
- Chemical formulas that give only the relative number of atoms of each type in a molecule are called empirical formulas.
- Once the empirical formula is known, additional experiments can give the information needed to convert the empirical formula to the molecular one. In addition, there are many substances that do not exist as isolated molecules. For these substances, we must rely on empirical formulas.
Picturing Molecules
The molecular formula of a substance does not show how its atoms are joined together.
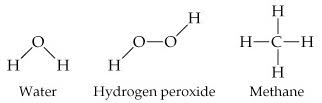
The atoms are represented by their chemical symbols, and lines are used to represent the bonds that hold the atoms together.
Perspective drawings use wedges and dashed lines to depict bonds that are not in the plane of the paper. This gives a crude sense of the three-dimensional shape of a molecule.
Ball-and-stick models show atoms as spheres and bonds as sticks. This type of model has the advantage of accurately representing the angles at which the atoms are attached to one another in a molecule.
Space-filling models depict what a molecule would look like if the atoms were scaled up in size.
2.7 ∣ Ions and Ionic Compounds
If electrons are removed from or added to an atom, a charged particle called an ion is formed. An ion with a positive charge is a cation (pronounced CAT-ion); a negatively charged ion is an anion (AN-ion).
- The net charge on an ion is represented by a superscript.
- In general, metal atoms tend to lose electrons to form cations and nonmetal atoms tend to gain electrons to form anions. Thus, ionic compounds tend to be composed of both metal cations and nonmetal anions, as in NaCl.
- It is important to realize that the chemical properties of ions are very different from the chemical properties of the atoms from which the ions are derived.
Predicting Ionic Charges
- The elements of group 8A are called the noble-gas elements. The noble gases are chemically nonreactive elements that form very few compounds.
The periodic table is very useful for remembering ionic charges, especially those of elements on the left and right sides of the table.
Ionic Compounds
- Sodium chloride, which we know better as common table salt, is an example of an ionic compound, a compound made up of cations and anions.
- Ionic compounds are generally combinations of metals and nonmetals, as in NaCl.
- The ions in ionic compounds are arranged in three-dimensional structures. Because there is no discrete “molecule” of NaCl, we are able to write only an empirical formula for this substance. This is true for most other ionic compounds.
- Because chemical compounds are always electrically neutral, the ions in an ionic compound always occur in such a ratio that the total positive charge equals the total negative charge.
2.8 ∣ Naming Inorganic Compounds
- The system used in naming substances is called chemical nomenclature, from the Latin words nomen (name) and calare (to call).
- Many important substances that have been known for a long time, such as water (H2O) and ammonia (NH3), do have traditional names (called common names).
- Organic compounds contain carbon and hydrogen, often in combination with oxygen, nitrogen, or other elements. All others are inorganic compounds.
Names and Formulas of Ionic Compounds
Cations
- Most metals that form cations with different charges are transition metals, elements that occur in the middle of the periodic table, from group 3B to group 2B.
- The metals that form only one cation (only one possible charge) are those of group 1A and group 2A, as well as Al3+ (group 3A) and two transition-metal ions: Ag+ (group 1B) and Zn2+ (group 2B).
The Hg2(2)+ ion is unusual because, even though it is a metal ion, it is not monatomic. It is called the mercury(I) ion because it can be thought of as two Hg+ ions bound together. The cations that you will encounter most frequently in this text are shown in boldface. You should learn these cations first.
| Common Cations | ||||
|---|---|---|---|---|
| Charge | Formula | Name | Formula | Name |
| 1+ | H+ | hydrogen ion | NH4 | copper(I) or cuprous ion |
| Li+ | lithium ion | Cu+ | copper(I) or cuprous ion | |
| Na+ | sodium ion | |||
| K+ | potassium ion | |||
| Cs+ | cesium ion | |||
| Ag+ | silver ion | |||
| 2+ | Mg2+ | magnesium ion | Co^2+ | cobalt(II) or cobaltous ion |
| Ca^2+ | calcium ion | Cu^2+ | copper(II) or cupric ion | |
| Sr^2+ | strontium ion | Fe^2+ | iron(II) or ferrous ion | |
| Ba^2+ | barium ion | Mn^2+ | manganese(II) or manganous ion | |
| Zn^2+ | zinc ion | Hg2^2+ | mercury(I) or mercurous ion | |
| Cd^2+ | cadmium ion | Hg^2+ | mercury(II) or mercuric ion | |
| Ni^2+ | nickel(II) or nickelous ion | |||
| Pb^2+ | lead(II) or plumbous ion | |||
| Sn2+ | tin(II) or stannous ion | |||
| 3+ | Al3+ | aluminum ion | Cr^3+ | chromium(III) or chromic ion |
| Fe^3+ | iron(III) or ferric ion |
Anions
a. The names of monatomic anions are formed by replacing the ending of the name of the element with -ide.
b. Polyatomic anions containing oxygen have names ending in either -ate or -ite and are called oxyanions.
Prefixes are used when the series of oxyanions of an element extends to four members, as with the halogens. The prefix per- indicates one more O atom than the oxyanion ending in -ate; hypo-indicates one O atom fewer than the oxyanion ending in -ite.
| Common Anions | ||||
|---|---|---|---|---|
| Charge | Formula | Name | Formula | Name |
| 1- | H^- | hydride ion | CH3COO− (or C2H3O2) | acetate ion |
| F^− | fluoride ion | ClO3 | chlorate ion | |
| Cl^− | chloride ion | ClO4^− | perchlorate ion | |
| Br^− | bromide ion | NO3^− | nitrate ion | |
| I^− | iodide ion | MnO4 | permanganate ion | |
| CN^- | cyanide ion | |||
| OH^− | hydroxide ion | |||
| 2- | O^2− | oxide ion | CO3^2− | carbonate ion |
| O2^2- | peroxide ion | CrO4^2- | chromate ion | |
| S^2− | sulfide ion | Cr2O7^2- | dichromate ion | |
| SO4^2− | sulfate ion | |||
| 3- | N^3- | nitride ion | PO4^3- | phosphate ion |
Ionic Compounds
Names of ionic compounds consist of the cation name followed by the anion name:
| CaCl2 | calcium chloride |
|---|---|
| Al(NO3)3 | aluminum nitrate |
| Cu(ClO4)2 | copper(II) perchlorate (or cupric perchlorate) |
Calcium bicarbonate is also called calcium hydrogen carbonate.
Names and Formulas of Acids
- Acids are an important class of hydrogen-containing compounds, and they are named in a special way.
- An acid is a substance whose molecules yield hydrogen ions (H+) when dissolved in water. An acid is composed of an anion connected to enough H+ ions to neutralize, or balance, the anion’s charge.
HOW TO NAME ACIDS
- Acids containing anions whose names end in -ide are named by changing the -ide ending to -ic, adding the prefix hydro- to this anion name, and then following with the word acid.
- Acids containing anions whose names end in -ate or -ite are named by changing -ate to -ic and -ite to -ous and then adding the word acid. Prefixes in the anion name are re- tained in the name of the acid.
2.9 ∣ Some Simple Organic Compounds
The study of compounds of carbon is called organic chemistry. Compounds that contain carbon and hydrogen, often in combination with oxygen, nitrogen, or other elements, are called organic compounds.
Alkanes
Compounds that contain only carbon and hydrogen are called hydrocarbons. In the simplest class of hydrocarbons, alkanes, each carbon is bonded to four other atoms.
Each alkane has a name that ends in -ane. The alkane with four carbons is called butane.
Some Derivatives of Alkanes
An alcohol, for example, is obtained by replacing an H atom of an alkane with an -OH group.
Alcohols have properties that are very different from those of the alkanes from which the alcohols are obtained. For example, methane, ethane, and propane are all color- less gases under normal conditions, whereas methanol, ethanol, and propanol are colorless liquids.
Compounds with the same molecular formula but different arrangements of atoms are called isomers.
Methane, ethane, and propane are the first alkanes, whereas methanol, ethanol, and propanol are the first alcohols. Chain length affects alkanes and alcohols. Octanes, eight-carbon alkanes, are normally liquids. Polyethylene, a solid used to build millions of plastic products including bags, food containers, and lab equipment, is produced by extending the alkane series to tens of thousands of carbon atoms.