IB Biology SL — Unit 4 Ecology
Page 1
Species, Communities, and Ecosystems
Species
Defined as groups of organisms capable of interbreeding to produce fertile offspring.
Can have autotrophic or heterotrophic nutrition methods.
Communities
Formed by populations of different species living together and interacting.
Ecosystems
Formed by communities interacting with the abiotic environment.
Autotrophs obtain inorganic nutrients from the environment.
Nutrient cycling maintains the supply of inorganic nutrients.
Sustainability and Classification
Ecosystems can be sustainable over long periods.
Classifying species based on their nutrition mode.
Setting up mesocosms to establish sustainability.
Testing species association using statistical methods.
Page 2
Species, Communities, and Ecosystems (Continued)
Reiteration of species, communities, and ecosystems definitions.
Nutrition Modes and Classification
Autotrophs synthesize organic molecules from inorganic sources.
Heterotrophs classified as consumers, detritivores, and saprotrophs.
Examples of different feeding patterns in heterotrophs.
Page 3
Species and Nutrition
Characteristics of species members and their ability to interbreed.
Challenges in defining species for organisms like bacteria and hybrids.
Modes of nutrition: autotrophs and heterotrophs.
Feeding Patterns
Classification of heterotrophs based on feeding habits.
Autotrophs produce their own organic molecules, while heterotrophs consume them.
Page 4
Detritivores and Saprotrophs
Detritivores obtain nutrients from decaying organic matter.
Saprotrophs digest dead organisms externally.
Mixotrophs and Sustainability
Some organisms can exhibit both autotrophic and heterotrophic nutrition.
Components required for ecosystem sustainability: energy availability, nutrient recycling, and waste management.
Nutrient Cycling
Nutrient cycling is essential to maintain nutrient availability in ecosystems.
Page 5
Autotrophs produce organic molecules from inorganic molecules
Consumers obtain organic molecules by consuming producers
Decomposers break down dead consumers' cells, returning nutrients to the soil
Mesocosm
Enclosed environments to observe a part of nature under controlled conditions
Used to test sustainability of nutrient cycles
Chi-Squared test
Determines species interactions in an environment
Positive association: predator-prey or symbiotic relationships
Negative association: competition for the same resources
No interaction: independent distribution
Page 6
Quadrat
A square measuring 1m on all sides
Helps count a small percentage of a sample
Used to estimate total number of organisms in a location
Applying Chi-Squared test to quadrat sampling data
Five steps: hypotheses, table of frequencies, chi-squared formula, degree of freedom, p-value
Page 7
Example of Chi-Squared Test Application
Testing association between ling and bell heather on moorland
Null hypothesis: no significant difference in species distribution
Alternative hypothesis: significant difference in species distribution
Constructing a table of frequencies for observed versus expected distribution
Page 8
Expected Results
Calculating expected distribution frequencies assuming random distribution
Calculating expected values based on probabilities
Checking calculated values against observed results in the table
Statistical Test: Observed and expected distribution of ling and bell heather
Page 9
Applying the chi-squared formula to calculate a statistical value
Determining the degree of freedom (df) for the test
Identifying the p-value to determine statistical significance
Page 10
Significance Level and Chi-Square Distribution
A value is significant if p < 0.05
Chi-square values for different probabilities
Critical value for df = 1 is 3.841
Niche
Definition and components of an ecological niche
Fundamental vs. realized niche
Page 11
Positive and Negative Associations
Predator-prey relationships and their impact on populations
Symbiotic relationships: mutualism, commensalism, parasitism
Competition types: intraspecific and interspecific
Page 12
Energy Flow in Ecosystems
Energy flow from sunlight to chemical energy
Energy transfer through food chains and heat loss
Energy storage in ATP and its usage
Energy Loss and Efficiency
Reasons for energy loss in organisms
Energy efficiency in living organisms
Pyramids of energy and their characteristics
Page 13
Biomass and Energy Conversion
Biomass definition and its relation to energy storage
Energy conversion forms: kinetic, electrical, light energy
Heat energy as a by-product in living organisms
Page 14
Biomass and Energy
Biomass as the total mass of organisms
Diminishing biomass along food chains
Final Note
Caution regarding the pyramid of numbers
Importance of understanding biomass and energy flow in ecosystems
Energy and Nutrient Movement in Ecosystems
Energy Flow in Ecosystems
Energy moves from one organism to another through feeding.
Arrows in food chains represent energy transfer.
Food chains start with producers and continue with consumers.
Food Webs
Represent complex feeding relationships in ecosystems.
Show how food chains are interconnected.
Organisms can have multiple food sources and predators.
Trophic Levels
Define the feeding position of organisms in a food chain.
Producers occupy the first trophic level.
Consumers occupy subsequent trophic levels.
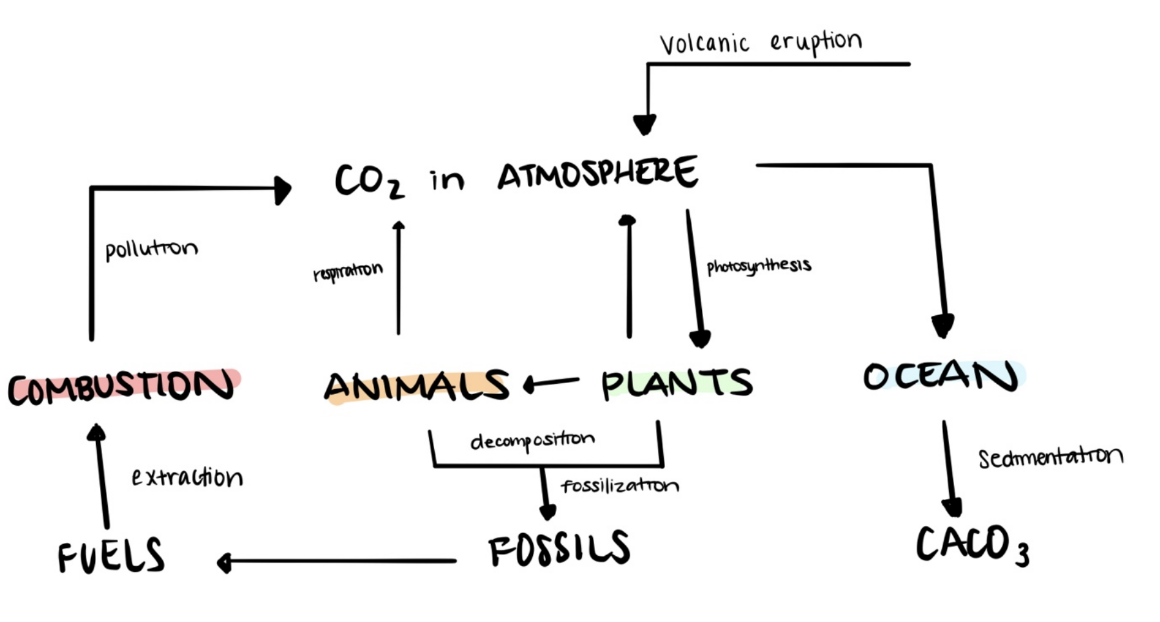
Carbon Cycling in Ecosystems
Carbon Cycle Overview
Carbon exchanges occur in the hydrosphere, biosphere, lithosphere, and atmosphere.
Various forms of carbon include atmospheric gases, oceanic carbonates, organic materials, and non-living remains.
Processes in Carbon Cycle
Autotrophs convert carbon dioxide into carbohydrates via photosynthesis.
Heterotrophs obtain carbon compounds through feeding.
Sources of CO2 production include combustion and respiration.
Carbon Cycle in Aquatic Ecosystems
Carbon exists as dissolved carbon dioxide and hydrogen carbonate ions.
Rising CO2 levels lead to acidic water, affecting organisms.
Impact on Climate Change
Consequences of Carbon Cycle Processes
Complete decomposition forms fossil fuels like oil and gas.
Incomplete decomposition leads to peat formation, releasing CO2 when burned.
Peat Formation and Fossil Fuels
Peat Formation
Peat forms in waterlogged soil due to partial decomposition.
Factors favoring peat production include organic matter, anaerobic, and acidic conditions.
Fossil Fuels
Oil and gas result from decay of marine organisms over millions of years.
Burning fossil fuels releases CO2 and other pollutants into the atmosphere.
Page 22
Combustion and Biofuels
Combustion:
Carbon dioxide produced by burning biomass and fossilized organic matter.
Organic compounds with hydrocarbons heated with oxygen undergo combustion, releasing energy, carbon dioxide, and water.
Biofuels:
Derived from living matter.
Advantages over fossil fuels:
Habitats not disrupted for mining.
Faster absorption of released carbon dioxide compared to fossil fuels.
Challenges:
Biofuels and bioenergy compete for finite land resources affecting food production and carbon storage.
Page 22
Methane Production
Methane:
Produced by methanogens in anaerobic conditions like wetlands, marine sediments, and ruminant animal digestive tracts.
Methane released into the atmosphere persists for about 12 years before oxidizing to carbon dioxide and water.
Page 23
Limestone and Carbon Sequestration
Limestone:
Majority made of calcium carbonate.
Formed by marine organisms absorbing carbon dioxide, which turns into calcium carbonate in their shells.
Bio-sequestration:
Process of removing carbon from the environment and locking it up.
Impact:
Over-mining limestone releases carbon dioxide back into the air, disrupting carbon sequestration.
Page 24
Climate Change and CO2 Emission
Greenhouse Gases:
Carbon dioxide and water vapor are significant greenhouse gases.
Impact depends on absorption of long-wave radiation and concentration in the atmosphere.
CO2 Concentration:
Human activities like deforestation, farming, and combustion increase CO2 levels.
Efforts to reduce reliance on fossil fuels are ongoing, promoting alternative energy sources.
Page 25
Greenhouse Gases and Impact
Greenhouse Gases:
Trap and hold heat in the atmosphere.
Water vapor and carbon dioxide have the largest warming effect.
Impact:
Determined by absorption capacity of long-wave radiation and concentration in the atmosphere.
Greenhouse Effect:
Earth's ability to retain heat and maintain moderate temperatures for life processes.
Page 26
Climate Change and Global Temperatures
Climate Change:
Greenhouse gases trap heat, leading to increased global temperatures.
Higher concentrations result in more extreme weather conditions and changes in circulating currents.
Vostok Ice Core:
Provides evidence of historical CO2 levels and temperatures.
Shows a positive correlation between CO2 concentrations and temperature.
Industrial Definitions:
Weather refers to current conditions, while climate pertains to long-term temperature and precipitation patterns.
Page 27
Industrial revolution increased fossil fuel use
Fossil fuel burning releases carbon dioxide, increasing atmospheric concentration
Trends related to fuel emissions, CO2 concentrations, and global temperatures
Strong positive correlation between fossil fuel emissions and CO2 levels
Atmospheric CO2 increased by ~38% since pre-industrial times
40% of CO2 emissions stayed in the atmosphere
Increase in CO2 correlates with global temperature rise
Consequences of greenhouse effect
Disease spread due to more temperate climates
Melting ice caps and permafrost
Extreme weather conditions
Extinction events due to climate change
Page 28
Consequences of enhanced greenhouse effect
Acidification of oceans
Rising sea levels displacing communities
Habitat destruction and expansion of temperate species
Global temperature rise effects on arctic ecosystems
Page 29
Relationship between atmospheric gases concentration and enhanced greenhouse effect
Ocean acidification
Oceans absorb a third of human CO2 emissions
CO2 solubility decreases as temperatures rise
Acidification threatens marine organisms and coral reefs
Page 30
Precautionary Principle
Calls for action when human activities pose environmental or health threats
Enhanced greenhouse effect requires precautionary measures due to complex climate changes
Onus for action lies on contributors to the enhanced greenhouse effect
Page 31
Action on climate change as a global issue involving various entities
Precautionary principle versus burden of proof
Arguments for and against action on climate change
Page 32
Diagrams to know: Carbon Cycle