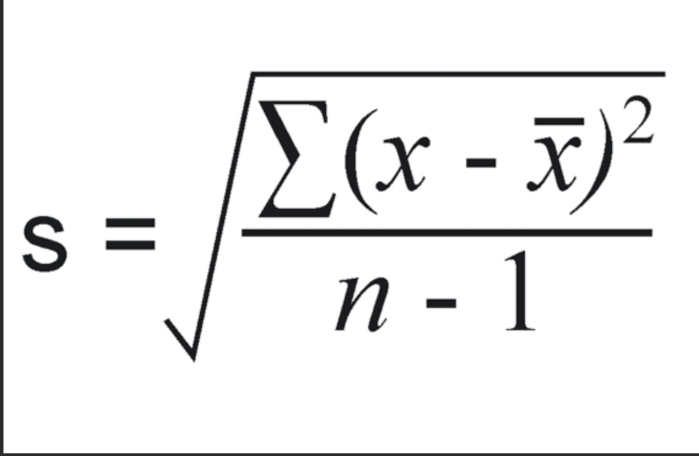Calculating Standard Deviation Steps:
Calculate mean = sum of the numbers divided by the total count of the numbers.
Take each number and subtract by the mean then square it (add them all together)
Divide that total number by the total count of numbers subtracted by 1
Square root the value
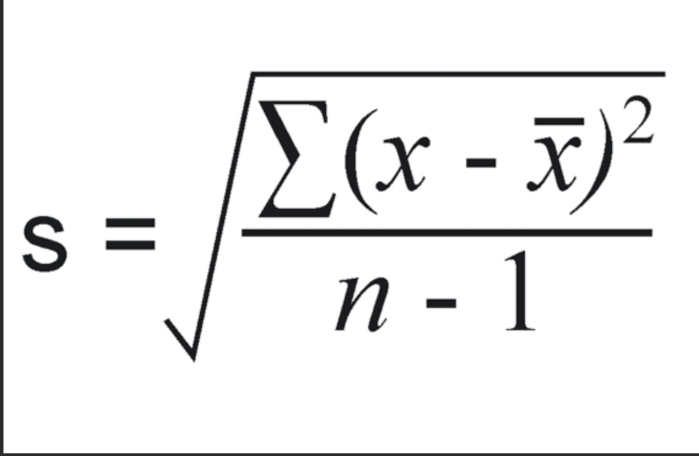
Formulas:
Density = Mass/Volume
Volume= Mass/Density
Energy = hv
wavelength symbol: λ (lambda)
Calculating concentration:
Calculating % cu mass in penny steps
1). Moles of Cu = concentration * Volume of element
2). Mass of Cu = Moles of Cu * molar mass of cu
3). (mass of cu / mass of penny)100%
Rydbergs constant = 1.097×10^7 m^-1
Emission of Light from Hydrogen and Metal Atoms:
Ultraviolet:
Highest Energy
Highest Frequency
Lowest wavelength
Infrared:
Visible light:
Lowest Energy
Lowest Frequency
Highest Wavelength
Isomers:
Cis
On the same side
Polar
Trans
On opposite sides
Nonpolar
 Note
Note Studied by 17702 people
Studied by 17702 people Note
Note Studied by 40 people
Studied by 40 people Note
Note Studied by 131 people
Studied by 131 people Note
Note Studied by 20 people
Studied by 20 people Note
Note Studied by 26 people
Studied by 26 people Note
Note Studied by 39 people
Studied by 39 people Knowt
Knowt