The Atmosphere
Layers of air surrounding Earth:
Composition of Atmosphere: 78% Nitrogen, 0.04% Carbon Dioxide, 20% Oxygen. Other trace gases are present in very small amounts, including argon, water vapor, and various pollutants.
Layers of the Atmosphere (Top to Bottom):
Thermosphere:
Extends from about 80 km (50 miles) to 600 km (373 miles) above the Earth's surface.
Contains a very thin atmosphere where temperatures can rise sharply, sometimes exceeding 2,500 °C (4,500 °F) due to solar activity.
This layer is where the auroras occur and where the International Space Station orbits; it absorbs high-energy solar radiation.
Mesosphere:
Ranges from about 50 km (31 miles) to about 80 km (50 miles) above the Earth's surface.
This layer is where most meteors burn up upon entering the Earth's atmosphere due to its increasing density.
Temperature decreases with altitude, reaching as low as -90 °C (-130 °F).
Stratosphere:
Lies between approximately 12 km (7 miles) and 50 km (31 miles) above the surface.
Contains the ozone layer, which absorbs and scatters ultraviolet solar radiation, protecting living organisms on Earth.
Temperature increases with altitude in this layer, which creates a stable atmospheric condition.
Troposphere:
Extends from the Earth's surface to about 12 km (7 miles) high and is the lowest layer of the atmosphere.
This layer contains most of the atmosphere's mass, including water vapor, and is where all weather phenomena occur.
Temperature decreases with altitude, with an average lapse rate of about 6.5 °C per kilometer.
The concentrations of molecules decrease in the atmosphere as you go up.
Weather:
Changes by the minute, hour, and day
What you get
Varies
Climate:
Long-term pattern of weather
What you expect
At least 30 years of date
Average
How climate and weather are measured:
Tree ring- determines the amount of rain that year. The larger the ring, the more rain that year.
Layers of coral- how warm the ocean was.
Layers of lake- thousands of years back. Isoptopes
Ice (O18)- Hundreds of years back. Ice Cores
Temperature follows CO2 concentration.
Energy Budget
How much energy (sun) the Earth is receiving and how much energy Earth is releasing. Radiation.
Electromagnetic waves: Light is NOT made of atoms, but photons
Electromagnetic spectrum (types of photons):
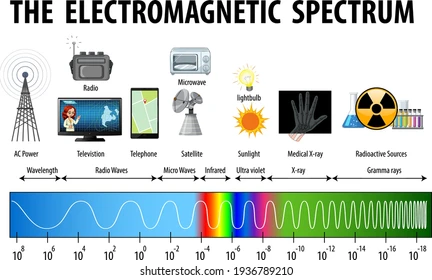
Between UV and infrared visible light, we can see colors.
Gasses in the atmosphere that block gamma rays from reaching the surface.
Radio waves make it to the surface.
Gamma rays are the deadliest.
Greenhouse effect
The greenhouse effect is a natural process that warms the Earth's surface. When the Sun's energy reaches the Earth, some of it is reflected in space, and the rest is absorbed and re-radiated by the Earth in the form of infrared radiation. Greenhouse gases in the atmosphere trap some of this infrared radiation, preventing it from escaping back into space.
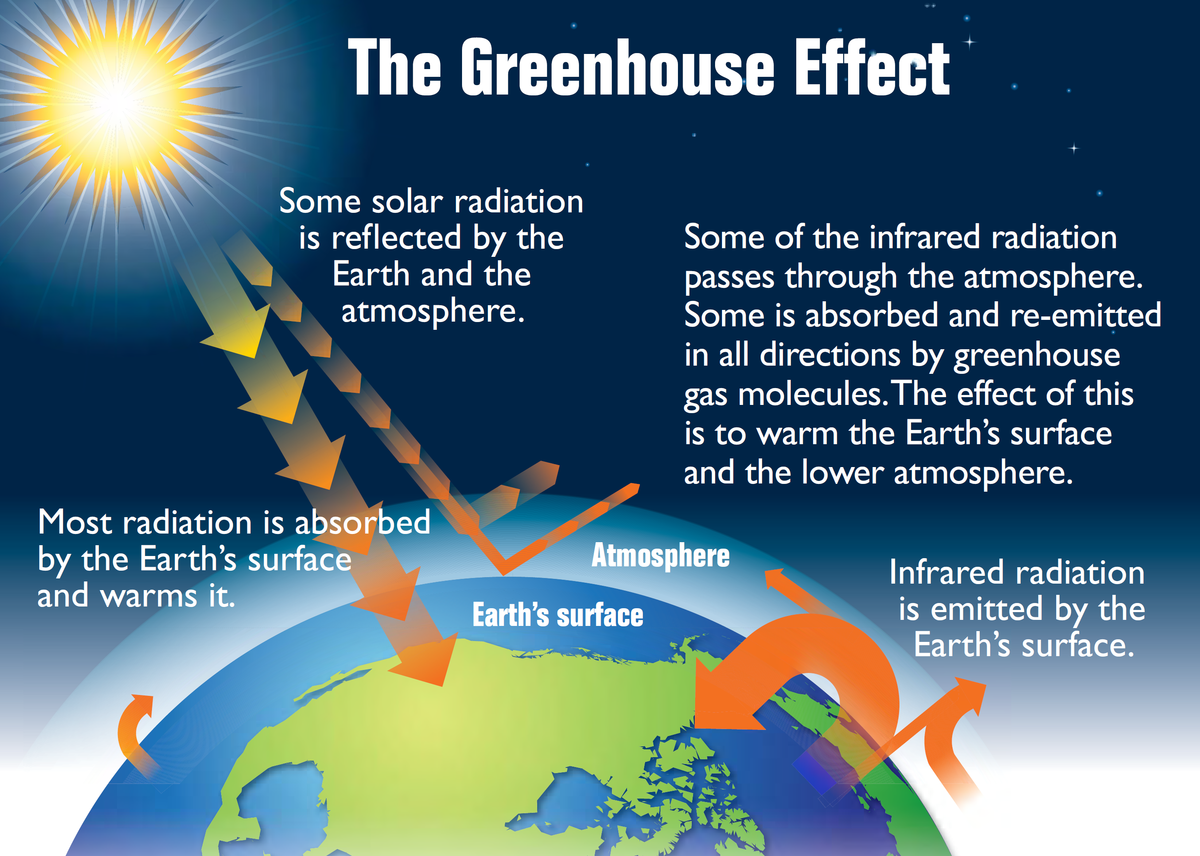
Greenhouse gasses:
Carbon Dioxide (CO2): Produced by burning fossil fuels, deforestation, and certain industrial processes. CO2 can linger in the atmosphere for thousands of years, contributing significantly to the greenhouse effect.
Methane (CH4): Released during the production and transport of coal, oil, and natural gas, as well as from livestock and other agricultural practices. Methane is over 25 times more potent than CO2 over a 100-year period.
Nitrous Oxide (N2O): Emitted from industrial activities, agricultural practices, and combustion of fossil fuels. While less abundant than CO2, it is approximately 298 times more effective than CO2 at trapping heat in the atmosphere.
Water Vapor: The most abundant greenhouse gas, it increases with temperature, making the greenhouse effect a feedback mechanism.
Ozone (O3): In the troposphere, it acts as a greenhouse gas, although it is also essential for filtering harmful UV radiation in the stratosphere.
Solar radiation: Larger area, spread out more. Smaller area (the equator) more radiation.
Atmospheric Air Currents
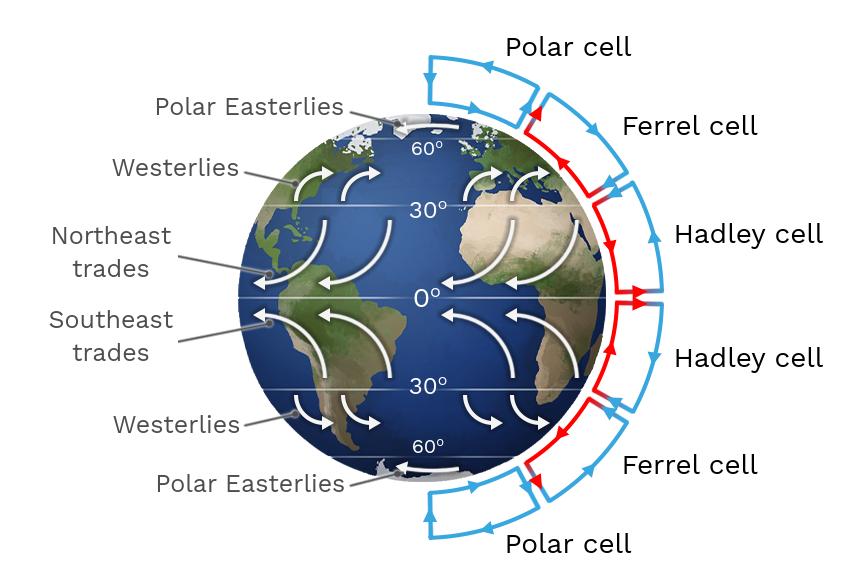
As air travels it contains moisture.
Rotates clockwise
Speed= distance/time
Types of cells determine the types of habitats:
Polar Cells ( 90 degrees, easterlies) ~ polar regions (ice).
Ferrel Cells ( 60 degrees, westerlies) ~ Forests
Hadly Cells (30 degrees, trades) ~ Deserts
Equator (0 degrees) ~ Forests
Ocean Current:
Largest current~ Theromhaline: driven by the differences in temperature (themro) and salinity (haline), which affects the density of seawater. This current plays a crucial role in regulating the Earth’s climate by transporting warm water from the equator toward the poles and cold water from the poles back toward the equator, influencing weather patterns and temperatures across the globe.
Air Pollution
Primary pollutant: Natural
Carbon Monoxide - CO
Carbon Dioxide - CO2
Nitrogen Oxides (NO)
N + O2 + heat = NO
Sulfur Dioxide - SO2
Lead- Pb
Secondary pollutant: Chemical reaction
Acid Decomposition
NO H2O HNO3
NO3 + =
SO2 O2 H2SO4
Particle Matter:
PM 2.5: combustion particles, organic compounds, metals, etc. Diameter < 2.5 mircometers.
PM 10: dust, mold, pollen, etc. Diameter < 10 mircometers
62% natural, 38% anthroprgenic
Volatile Organic Compounds (VOCS): synthetic man-made chemicals. Ex. paint, vehicle exhaust.
Ozone (O3) - At ground level a secondary pollutant.
Nox + VOC + Heat and Sunlight = ozone
Atmospheric Problems
Clean Air Act 1963: enacted to monitor, study, and control air pollution.
1965- Motor vehicle air pollution control act
1970- expanded sources covered and enforcement
1990- acid rain, ozone hole, more enforcement
CFC: Chlorfluorcarbon- man-made chemcials, such as hairspray. Destroys the ozone layer by breaking molecules apart.