CHEM 131 Midterm 1 Notes
Lecture 1
/
Dalton’s Theory of the Atom (1803):
Elements are made up of tiny, indestructible particles called atoms.
Atoms of one element cannot change into other atoms of another element — they can only change the way they are bound with other atoms
All atoms of a given element have the same mass and have properties that distinguish them from atoms of other elements
Atoms combine in whole ratios to form compounds
Properties of Protons:
Mass: 1.67262 × 10^-27 kg
Mass: 1.00727 amu
Relative Charge: 1+
Properties of Electrons:
Mass: 0.00091 × 10^-27 kg
Mass: 0.00055 amu
Relative Charge (1-)
Properties of Neutrons:
Mass: 1.67493 × 10^-27 kg
Mass: 1.00866 amu
Relative Charge: 0
Isotopes:
Isotopes are elements that have the same number of protons but different numbers of neutrons (ex: Carbon12)
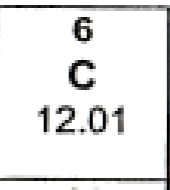
Parts of Periodic Table:
6 = atomic number (Z)
C = element symbol
12.01 = atomic weight
Electrons and Ions
Ions are charged atoms
The number of electrons in a neutral atom is equal to the number of protons in its nucleus
In chemical changes, however, atoms can lose or gain electrons and become charged particles called ions
Positively charged ions are called cations
Metal elements (Na+)
Negatively charged ions are called anions
Nonmetal elements (F-)
Lecture 2
Dalton’s Model of the Atom:
Elements are made up of tiny, indestructible particles called atoms.

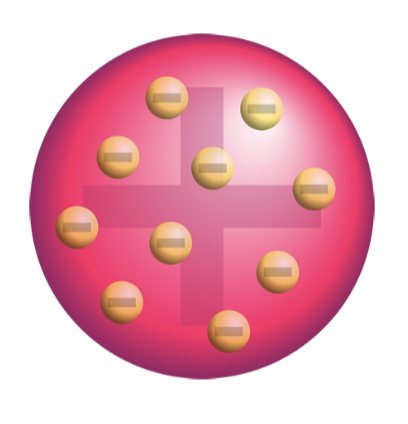
The Plum Pudding Model of the Atom:
Atoms contain negatively charged particles called electrons
Electrons exist in a positively charged space
The Nuclear Model of the Atom:
Most of the atom’s mass and all of its positive charge are contained in a small core called the nucleus
Most of the volume of the atom is empty space, throughout which tiny negatively charged electrons are dispersed
There are as many negatively charged electrons outside the nucleus as there are positively charged particles (protons) inside the nucleus —> keeps the atom electrically neutral
Also neutral particles within the nucleus (neutrons)
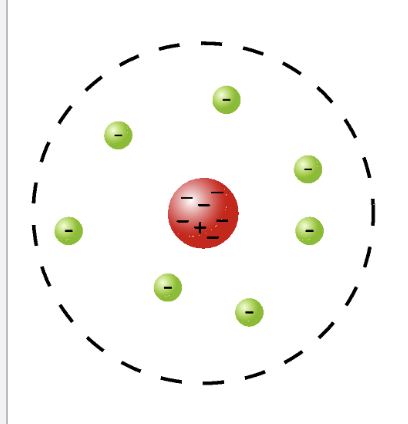
The Bohr Model of the Atom
Nucleus remains the same (p+, n0)
The electrons (e-) travel in orbits that are at a fixed distance from the nucleus
These orbits correspond to discrete/quantized energy levels
Electrons release energy when they transition from an orbit with higher energy down to an orbit with lower energy (we see that energy as light)
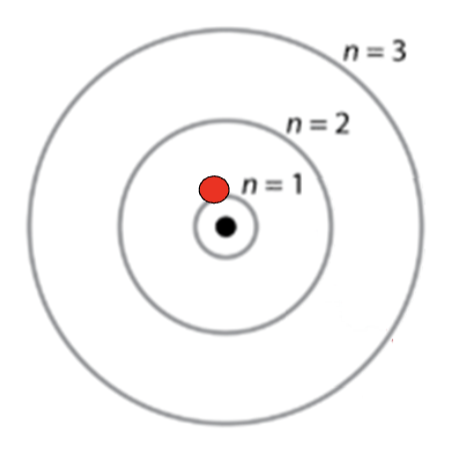
The Quantum Mechanical Model of the Atom
Nucleus remains the same (p+,n0)
The electrons do not travel in orbits
Electrons exist in probability domains that predicts their position ~95% of the time
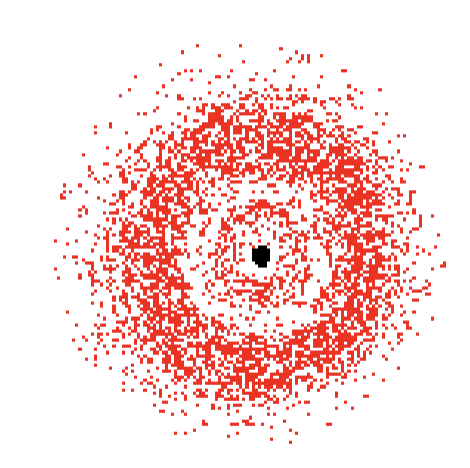
Electrons are considered to be neither a particle nor a wave (have both characteristics)
Wave nature: interference pattern
Particle nature: position, which slit is it passing through
Schrodinger’s Equation:
allows us to calculate the probability of finding an electron with a particular amount of energy at a particular location in the atom
The resulting value describes a probability domain in which can electron can be found ~95% of the time
Lecture 3
Think of quantum numbers as a hotel:
“n” - floors
“l” - rooms
“ml” - numbers of rooms of a type
“n” - principal quantum number (energy level)
n = integer = 1,2,3,4,5,6,7…
example: n=2, means electron is in the second energy level
higher n value, higher energy level
increased size of probability domain (location of electron) with a larger n value
Energy Levels in a Hydrogen Atom
Lyman Series: produced when electrons in a hydrogen atom transition from higher energy levels to the lowest energy level (n=1); emits photons of specific wavelengths
Balmer Series: produced when electrons in a hydrogen atom transition from higher energy levels to the n=2; emits photons of specific wavelengths
Passion Series: produced when electrons in a hydrogen atom transition from higher energy levels to the n=3; emits photons of specific wavelengths
Higher energy level to lower energy level - releases energy
Lower energy level to higher energy level - absorbs energy
“l” - angular momentum quantum number (orbital type)
l=0 - s orbital (spherical)
l=1 - p orbital (dumbbell)
l=2 - d orbital (clover leaf)
l=3 - f orbital (2-4 leaf clovers)
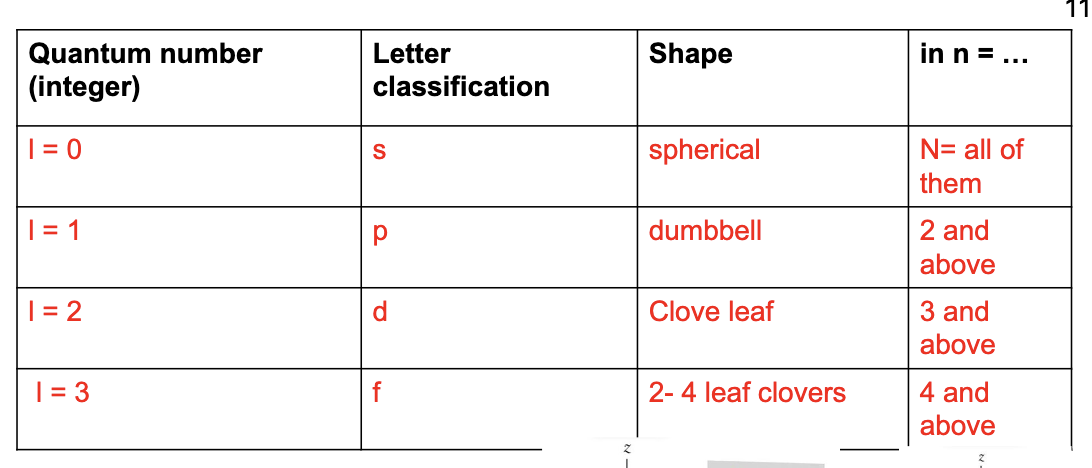
ml - magnetic quantum number (which orbital/orientation/subshell)
Lecture 4
l=0 (s), ml=0
l=1 (p), ml=-1,0,1
l=2 (d), ml=-2,-1,0,1,2
l=3 (f), ml=-3,-2,-1,0,1,2,3
s-orbital:
n=1 and up
l=0
ml=0
p-orbital:
n=2 and up
l= 1
ml=-1,0,1,
d-orbital:
n=3 and up
l=2
ml=-2,-1,0,1,2
f-orbital:
n=4 and up
l=3
ml=-3,-2,-1,0,1,2,3
Pauli Exclusion Principle: no two electrons can have the same four quantum numbers (no more than two electrons can occupy the same orbital and the two electrons in the same orbital must have opposite spins)
An electron can be found in the lowest energy orbital available (ground state) 95% of the time.
The orbital lowest in energy is whichever orbital can get electrons the closest to the nucleus.
Coulomb’s Law
Particles of like charge repel (so that energy lowers when they separate)
Particles of unlike charges attract (so that energy lowers when they get closer together)
The larger the charges, the larger the energetic effects are
Z-effective: the total amount of attraction that an electron feels for the nucleus’s protons is called the effective nuclear charge (Zeff) of the electron
Shielding: inner electrons of an atom block the positive charge of the nucleus from reaching the outer charge —> inner electrons “shield” the outer electrons from the full nuclear charge (causes lower Zeff)
Penetration: the shape of orbitals allow electrons the possibility to get closer to the nucleus, which lowers its energy overall (if an outer electron had the possibility to get closer to the nucleus it would take it)
Lecture 5
Aufbau Principle: electrons enter (e.g. fill) atomic orbitals from lowest energy to highest
Hund’s Rule: Electrons will always enter empty orbitals before they pair up
Ex: find the valence and core electrons of the following electron configurations (try looking at n and l once you got to d)
Sodium: 1s2,2,s2,2p6,3s1
Valence: 1
Core: 10
Bromine: 1s2,2s2,2p6,3s2,3p6,4s2,3d10,4p5
Valence: 7
Core: 28
Calcium: 1s2,2s2,2p6,3s2,3p6,4s2
Valence: 2
Core: 18
Phosphorus: 1s2,2s2,2p6,3s2,3p3
Valence: 5
Core: 10
Titanium: 1s2,2s2,2p6,3s2,3p6,4s2,3d2
Valence: 4
Core: 18
When electrons become ions, they gain or lose electrons to achieve a full valence shell (2 or 8 electrons)
Electrons are gained from the valence shell to fill it
Electrons are lost from the valence shell to empty it and unveil the full shell underneath
Typically, metals will lose electrons and non-metals will gain electrons.
Lecture 6
Writing Elements as Ions:
Sodium: 1s2,2s2,2p6,3s1
Na+ (lose one electron to get to a full outer shell)
Bromine: 1s2,2s2,2p6,3s2,3p6,4s2,3d10,4p5
Br- (gain one electron to get to a full outer shell)
Phosphorus: 1s2,2s2,2p6,3s2,3p3
P³-
Titanium: 1s2,2s2,2p6,3s2,3p6,4s2,3d2
Ti4+ (an exception; it has multiple charges, with the preferred being 4+)
The Periodic Law: elements with similar properties recur in a regular patter (fall into columns)
The quantum-mechanical model explains this because the number of valence electrons and the types of orbitals they occupy are also periodic
Alkaline Earth Metals: Column 2A
Alkali Metals: Column 1A (except for hydrogen)
Halogen: Column 17
Noble Gases: Column 18
Transition Metals: Column 3-12
Other Nonmetals: Hydrogen + elements above staircase (B—> O —> Te —> B)
Other Metals: Below Staircase (Al —> Po —> Lv —> Nh—→ Al)
Noble Gases:
Full valence shells
particularly unreative and stable
Halogens:
One elctron short of full valence shell
Tend to gain one electron to form 1- ion (ex: F-)
Alkali Metals:
one electron beyond full valence shell
tend to lose one electron and form 1+ ion (ex: Na+)
Alkaline Earth Metals:
two electrons beyond full valence shell
tend to lose two electrons and form 2+ ion (ex: Ca2+)
s and p block, row number = n
d block, n= row number -1
f block, n= row number -2
Electron Configuration Exceptions:
Mo - [Kr] 5s1, 4d5
Cr - [Ar] 4s1, 3d5
Cu - [Ar] 4s1, 3d10
Ag - [Kr] 5s1, 4d10
Au - [Xe] 6s1,4f14,5d10
Electron configurations arise due to some elements having more stability with half-filled (like d5) and fully filled (like d10) orbitals. They reduce electron repulsion and create a more balanced, lower-energy arrangement of electrons.
Characteristics of atoms (and ions) which can be explained based on their position on the periodic table and either their Zeff (effective nuclear charge) or their n (principal quantum number)
Effective Nuclear Charge and Periodic Trends
Z is the nuclear charge, and S is the number of electrons in lower energy levels
Electrons in the same energy level contribute to screening, but since their contribution is so small, they are not part of the calculation.
Zeffective = Z (atomic number) - S (number of core/shielding electrons)
Examples:
Potassium: 1s2,2s2,2p6,3s2,3p6,4s1
Zeffective = 19 - 18 = +1
Chlorine: 1s2,2s2,2p6,3s2,3p5
Zeffective = 17 - 10 = +7
Lecture 7
Z-effective: effective nuclear charge, essentially how much of the nucleus’s positive charge an electron feels after considering the shielding effects of other electrons in an atom
Shorthand for Z-effective:
Only for neutral atoms, not ions
Atomic radius
distance between nucleus and valence electrons
as we move down the periodic table, we are adding another energy level, and therefore valence electrons are getting farther from the nucleus
ex: Na has smaller atomic radii than nucleus
atomic radii also increases as we go to the left of the periodic table
higher the Zeff, the more pull towards the nucleus, and the smaller the radii is going to be (higher Zeff on the right side of the periodic table)
Ionic Radii: Cations
Example: K+= 1s2,2s2,2p6,3s2,3p6
Zeff= 19-10 = +9
Cations are smaller than their neutral counterparts
Ionic Radii: Anions
Example: Cl- = 1s2,2s2,2p6,3s2,3p6
Zeff= 17 - 10 = +7
Anions are larger than their neutral counterparts
Isoelectronic species: Ions or atoms that have the same number of electrons or a similar electronic structure
Ex: Neon (10 electrons) and Na+ (10 electrons)
Atoms with different atomic numbers (z) but same electron configuration
Can only compare Zeff among isoelectronic species
The species with the largest positive charge is the smallest (the highest Zeffective is going to have the smallest radii)
relationship between z-effective, radii, and ions flashcard
Ionization energy (IE)
the amount of energy required to remove an electron from an atom in its gaseous state
Energy is required → E+, formation of cation
Na(g) → Na+(g) + e-
IE1 = +496 KJ
Na+(g) → Na2+(g) + e-
IE2 = 4560 KJ
Ionization energy are successive
Ie1 (first electron), Ie2 (second electron), Ie3 (third electron)…
Ionization energy increases as we go to the right of the periodic table and up the periodic table
Electrons on the right hold on tighter to stay closer to the nucleus
Ionization energy increases with each successive removal of the outermost electron
Ionization energy and atomic radius are opposite in their trends.
Electron affinity (EA)
The energy change associated with the gaining of an electron by an atom in its gaseous state
measure of how easily an atom will accept an additional electron
electron affinity is usually (not always) negative because an atom or ion releases energy when it gains an electron (exothermic)
Noble gasses EA are always +
electron affinity gets more negative (excluding noble gasses) as you go from left to right
Lecture 7a
Why do atoms react to form molecules?
to achieve full valence shells
H, He → 1s2
Two ways that atoms will achieve a full valence shell:
Ionic: one atom will give an electron(s); another atom with take it/them
Covalent: two atoms will share electrons
Ionic Bonding
not quite a bond, but an attraction
ion-ion (electrostatic) interaction
between a metal and a nonmetal
electrons will be lost and gained
metal → cation (+)
nonmetal → anion (-)
Example: NaCl → sodium loses an election (Na+) and Cl gains an electron (Cl-)
oppositely charged ions are held together by ionic bonds, forming a crystalline lattice.
Covalent Bonds:
occur between two or more nonmetals. the atoms share electrons between them, composing a molecule.
covalently bonded compounds are also called molecular compounds
example - bond between nitrogen and oxygen (both nonmetals)
Naming Ionic Compounds
Ionic compounds can be categorized into two types, depending on the metal in the compound
with naming ionic compounds, no need to look at subscript
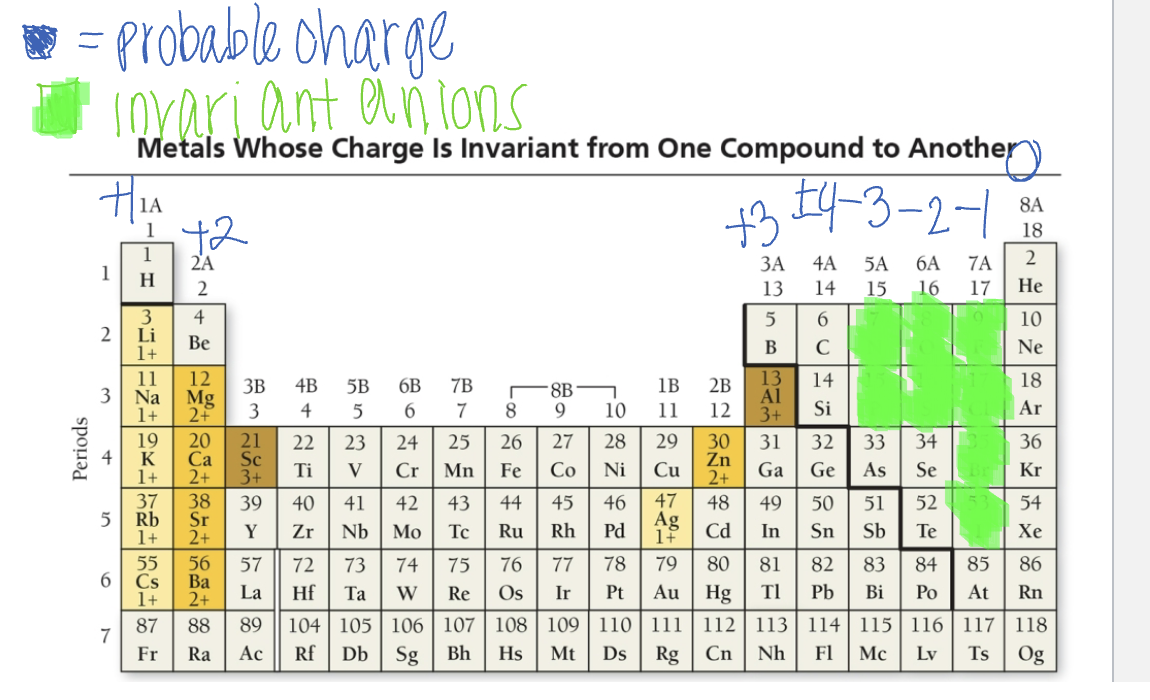
Invariant: metals whose charge remains the same when forming an ionic bond
alkali except for hydrogen and Fr (1+), alkaline earths except for Be and Ra (2+), zinc (2+), Al (3+) and Ag (1+)
Variant: metals that can vary in charge from one compound to another
transition metals, inner transition metals, and p-block metals
Binary compounds: contain only two different elements.
naming of binary ionic invariant compounds:
name of cation (metal) + base name of anion (nonmetal) + ide
naming of binary ionic variant compounds:
name of cation is followed by a roman numeral (in parentheses) that indicates the charge of the metal in that particular compound + base name of anion (nonmetal) + ide
example: Cu2O → copper (II) oxide
example: CuO → copper (I) oxide
Developing Formulas from Names of Invariant Charge:
1) write each species in name as a neutral atom
2) determine the charge that they would have once they fill their valence shell
3) cross # portion of charges
example: Calcium Chloride
Ca, Cl
Ca 2+, Cl- (cross)
CaCl2
example: Magnesium Oxide
Mg, O
Mg 2+, O2- (cross)
MgO (simplify the 2,2)
example: sodium nitride
Na, N
Na 1+, N 3-
Na3N
Developing Formulas from Names of Variant Charge:
Example: Cu2O
to name this, uncross the subscripts to get the charges
Cu 1+, O2-
Copper (I) Oxide
Example: CuO
Cu 2+, O 2-
Copper (II) Oxide
Example: Co2O3
Co 3+, O 2-
Cobalt (III) Oxide
Example: CoO2
Co 4+, O 2-
Cobalt (IV) Oxide
Example: PdCl2
Pd 2+, Cl-
Palladium (II) Chloride
Example: FeN
Fe 1+, N 1-
Fe 3+, N 3-
Iron (III) Nitride
Iron (III) Sulfide
Fe, S
Fe 3+, S 2-
Fe2S3
Titanium (IV) Carbide
Ti, C
Ti 4+, C 4-
TiC
Gold (III) Iodide
Au, I
Au 3+, I -
AuI3
Common Anions to Memorize:
F-
Cl-
Br-
I-
O2-
S2-
N3-
P3-
Metals with Variant Charge to Memorize:
Cr 2+ - Chromium (II)
Cr 3+ - Chromium (III)
Fe 2+ - Iron (II)
Fe 3+ - Iron (III)
Co 2+ - Cobalt (II)
Co 3+ - Cobalt (III)
Cu + - Copper (I)
Cu 2+ - Copper (II)
Sn 2+ - Tin (II)
Sn 4+ - Tin (IV)
Hg2 2+ - Mercury (I)
Hg 2+ - Mercury (II)
Pb 2+ - Lead (II)
Pb 4+ - Lead (IV)
Polyatomic Ions:
Ions that contain more than one atom
Polyatomic ion will replace the metal (in the case of ammonium, NH4+) or replace the nonmetal
Oxyanions: most polyatomic ions are oxyanions, anions containing oxygen and another element
the one with more oxygen atoms has the ending -ate
No3- is nitrate
the one with fewer oxygen atoms has the ending -ite
No2- is nitrite
If there are more than two ions in the series, then the prefixes hypo- (less than) and per- (more than) are used
Hypochlorite: ClO -
Chlorite: ClO2 -
Clorate: Clo3 -
Perchlorate: ClO4 -
Polyatomic Ions to Memorize:
Acetate: C2H3O2 -
Carbonate: CO3 2-
Hydrogen Carbonate (bicarbonate): HCO3 -
Hydroxide: OH -
Nitrite: NO2 -
Nitrate: NO3 -
Chromate: CrO4 2-
Dichromate: Cr2O7 2-
Phosphate: PO4 3-
Hydrogren Phosphate: HPO4 2-
Dihydrogen Phosphate: H2PO4 -
Ammonium: NH4 +
Perflourate: FO4 -
Flourate: FO3 -
Flourite: FO2 -
Hypoflourite: FO -
Permanganate: MnO4 -
Sulfite: SO3 2 -
Hydrogen sulfite (or bisulfite): HSO3 -
Sulfate: SO4 2-
Hydrogren sulfate (or bisulfate): HSO4 -
Cyanide: CN -
Peroxide: O2 2-
Oxyanions to Memorize:
Hypochlorite: ClO -
Chlorite: ClO2 -
Chlorate: ClO3 -
Perchlorate: ClO4 -
Perbromate: BrO4 -
Periodate: IO4 -
Bromate: BrO3 -
Iodate: IO3 -
Bromite: BrO2 -
Iodite: IO2 -
Hypobromite: BrO -
Hypoiodite: IO -
Naming Binary Ionic Compounds with Polyatomic Ions
uncross the subscripts to get the charges
Example: Na2(SO4)
Na 2+, (SO4) 2-
Na(SO4)
Sodium Sulfate
Example: Pb(NO3)2
Lead, (NO3) -2
Lead (II) Nitrate
Example: (NH4)2S
Ammonium, Sulfur
Ammonium +2, Sulfur -2
Ammonium Sulfide
Example: Mn(CO3)
Manganese, Carbonate (2-)
Manganese (II) Carbonate
Example: Copper (II) Hydroxide
Cu 2+, OH -
Cu(OH)2
Covalent Compounds: Formulas and Names
The formula for a covalent compound cannot readily be determined from its constituent elements because the same combination of elements may form many different molecular compounds, each with a different formula.
example of N and O: NO, NO2,N2O, N2O3, N2O4, N2O5
Covalent compounds are composed of two or more nonmetals
Naming Covalent Compounds:
Write the name of the element with the smallest group first
if the two elements lie in the same group, then write the element with the greatest row number first
The prefixes given to each element indicate the number of atoms present
prefix + name of first element + prefix + base name of 2nd element + ide
Prefixes for Covalent Compounds to Memorize:
Mono = 1
Di = 2
Tri = 3
Tetra = 4
Penta = 5
Hexa = 6
Hepta = 7
Octa = 8
Nona = 9
Deca = 10
Acids:
Acids are molecular compounds that release hydrogen ions (H+) when dissolved in water.
Acids are composed of hydrogen, usually written first in their formulas, and one or more nonmetals, written second.
Binary acids have H+ cation and nonmetal anion
Hydro + base name of nonmetal + -ic + acid
Example: HCl
Hydrogen, Chlorine
Hydrochloric Acid
Example: H2Se
Hydrogren, Selenium
Hydroselenic Acid
Oxyacids have H+ cation and polyatomic anion
base name of oxyanion + -ic + acid
Example: HNO3
Nitric Acid
Example: H2SO4
Sulfuric Acid
Acids - formula has H as first element
Binary acids contain only two elements
Oxyacids contain oxygen
Electronegativity: measure of how well an atom can attract an electron to itself
